© Borgis - New Medicine 2/2004, s. 26-33
Dimitrous G. Panoussopoulos MD, PhD, S.D. Panoussopoulos MD
Bile duct cancer
Propaedeutic Surgery Clinic, Medical University of Athens, Athens, Greece
Summary
Bile duct cancer is a rare malignancy occuring in a biliary tree. Although the rate of occurence is low it is still worthy to be disscussed. Cholangiocarcinoma is a diagnosis in only 5 to 10% of all periampullary tumors. Diagnosis, staging, classification and and prognostic factors are presented.
Authors describe two kinds of treatment: surgical treatment and palliative therapy pointing out objectives, criteria and clinical outcomes of each. Adjuvant therapy seems to be no effective for cholangiocarcinoma.
EPIDEMIOLOGY – CLASSIFICATION
Bile duct cancer represents a rare malignancy that can occur anywhere along the intrahepatic or extrahepatic biliary tree. It comprises less than 2% of all cancer diagnoses (1). The overall rate of occurrence is 1.2/100,000 people, with 2/3 of all cases developing in patients over 65 years of age (2). Over 95% are epithelial adenocarcinomas, also known as "cholangiocarcinomas”, predominating in the extrahepatic biliary tree. Men are affected slightly more than women and usually later in life (3). Most classification systems have separated intrahepatic from extrahepatic tumors and the latter have been traditionally subdivided into 3 groups, based on anatomical location (4). Upper third or hilar tumors are those located in the common hepatic duct and/or the right and left hepatic ducts including their confluence. Middle third tumors develop in the region between the upper border of the duodenum and extend to the common bile duct. Lower third or distal tumors arise between the anpulla of Vater and the upper border of the duodenum (4). Intrahepatic cholangiocarcinoma are rare neoplasms, comprising 6 to 10% of all cholangiocarcinomas, and they typically present as solitary hepatic masses (5). Tumors at the biliary confluence at the hilum of the liver (so-called Klatskin tumors) are the most frequently encountered and hilar cholangiocarcinomas (HCCA) comprise approximately 60% of the total (6). Middle and distal third tumors comprise about 20% each of all cholangiocarcinomas (6). Cholangiocarcinoma is also the diagnosis in only 5 to 10% of all periampullary tumors with ampullary, duodenal and pancreatic cancers being the rest of them. A small percentage of patients (<10%) may present with diffuse tumors of the entire extrahepatic bile duct and in such cases, surgery is never curative and prognosis is very poor (7). According to many authorities, the spectrum of extrahepatic cholangiocarcinomas is best classified into two broad groups: hilar or perihilar (HCCA) and distal (4). This is due to the fact that middle third lesions are relatively rare and are usually managed either as a proximal lesion with hilar resection or as a distal lesion with pancreatoduodenectomy. The classification to HCCA and total bile duct carcinoma correlates better with anatomic distribution and implies preferred treatment strategies: supraduodenal bile duct excision, portal lymphadenectomy, cholocystectomy, bilioenteric reconstruction and, in many cases, partial hepatectomy for HCCA and pancreaticoduodenectomy, or local bile duct excision for total cholangiocarcinomas (4).
CLINICAL PICTURE
Painless jaundice occurs in 90% of patients. Additional symptoms may be weight loss (30%), abdominal pain (20%), fever (10%) (8). Itching is the most commonly reported symptom occurring in up to 30% of patients and may precede the development of jaundice. Jaundice may be absent in the case of an intrahepatic cholangiocarcinoma or an HCCA that involves only the right or left hepatic duct. If the tumor spreads towards the portal vein, ipsilateral lobar atrophy can develop, in which case an isolated alkaline phosphatace elevation may be the only clinical finding.
Physical examination might reveal a moderate liver enlargement. The gallbladder can be palpable in distal tumors (Courvoisier´s sign). Ascites is rare. Advanced tumors obstructing the portal vein can lead to portal hypertension and splenomegaly (3).
Although cholangitis is a rare initial presentation, the rate of both its occurrence and that of bactobilia increases significantly with either endoscopic or percutaneous biliary tract instrumentation. The usefulness of stents in the asymptomatic jaundice patient is controversial. A two-fold increase in postoperative infections complications have been associated with preoperative stenting (9). Increased morbidity has been associated with preoperative biliary stenting in patients undergoing either biliary duct excisions or pancreaticoduodenectomy for cholangiocarcinomas (10, 11, 12). On the other hand, several authors have reported the usefulness of biliary stenting, which may provide several advantages such as normalization of coagulation function, reduction of circulating endotoxins, improvement of renal and immune function, and the most important one – facilitate the intraoperative identification of the right and left hepatic ducts at the case of the liver (3, 13).
PATHOLOGY &NDASH; TUMOR BIOLOGY
Certain pathological conditions related to acute or chronic biliary epithelial injury appear to predispose to the development of cholangiocarcinomas 40% of autopsy specimens and 10% to 30% of liver explants after their transplantation from patients with primary sclerosing cholangitis (PSC) contained occult foci of cholangiocarcinoma (14). It seems, that PSC and cholangiocarcinoma may represent a spectrum of the same disease process. Another group of patients carrying an increased risk for cholangiocarcinoma are those with cystic disease of the biliary system in the form of choledochal cysts or Caroli´s disease, due to a chronic biliary inflammation associated with those conditions (7). Biliary tract infection with parasites endemic to Southeastern Asia, such as Clonorchis sinensis and Opisthorchis viverrin, chronic cholelithiasis, hepatolithiasis and previous cholecystectomy are also accounted among the risk factors for the development of these neoplasms (8). Chronic typhoid carriers and exposure to asbestos, thorium dioxide and nistrosamines are less well-documented risk factors (8).
Based on its macroscopic appearance, cholangiocarcinoma is subclassified into the following types: a.the papillary variant (about 10%), which has a tendency for multicentricity, is most common in the mid to distal bile duct and carries a more favorable prognosis, b.the nodular variant, which occurs most commonly in the upper and mid bile duct and usually presents as a fibrotic mass, c.the selerosing variant (about 70%), mainly encountered at the hilum and appears as an annular thickening of the duct wall with both longitudinal and radial tumor infiltrations, d. the rare diffuse variant presents with extensive involvement of the entire extrahepatic bile duct, e.the rare carcinoid tumors, and f.the tumors that metastatically involve the biliary tree, originating usually from colorectal cancers.
Hematogenous spread of HCCA is rare, while lymph node metastasis may be present up to 1/3 of the cases (15). Extensive subepithelial tumor spread beyond the gross tumor margin is also common and longitudinal spread may extend 15 mm to 20 mm proximally and 5 mm to 10 mm distally (16).
DIAGNOSIS &NDASH; EVALUATION
The objectives of preoperative evaluation of a patient with suspected cholangiocarcinoma are as follows: a.assessment of the extent of biliary tract and portal vein, b.assessment of liver function and status (lobular atrophy, concommitent liver pathology), c.evaluation of nodal and/or distant metastasis, and d.assessment of patient´s general status and tolerability for an operation.
Investigative radiology initially includes noninvasive studies such as U/S and CT, which are performed to generally assess the locoregional extent of tumor and the presence of metastases (3). U/S, especially when combined with duplex scanning, has an accuracy of over 50%. CT scans are also highly accurate at imaging small lesions and identifying their relationship to the portal triad and hepatic parenchyma. Delayed images after intravenously contrast-enhanced spiral CT scans are the most sensitive, since cholangiocarcinomas tend to return the contrast longer than adjacent normal tissue (3). In selected cases, a celiac and superior mesenteric arteriogram with late-phase portography may be useful to assess resectability, although not much utilized nowadays. Instead, when duplex U/S fails to reveal vascular involvement by tumor, operation is favored as the principal determinant of resectability (3). The efficacy of magnetic resonance cholangiopancreatography (MReP) has been already well documented and has largely replaced invasive methods (percutaneous and endoscopic cholangiography), especially among the advocates of limitation of preoperative biliary instrumentation (17).
Nevertheless, according to most authorities, cholangiography is mandatory when noninvasive studies suggest malignant biliary obstruction (3). Percutaneous transhepatic cholangiography (PTC) is the most useful and definitive radiologic test for bile duct cancer (3). It outlines the tumor and proximal biliary system and permits external drainage catheters placement above the lesion. Intraoperatively, these catheters assist in the identification of the proximal biliary tract and may be replaced by larger silastic transanastomotic stents if necessary (3). If resection or operative bypass are not possible, they can also be internalized to the duodenum or, less often, left to external drainage to alleviate the jaundice (3). ERCP, with or without endoscopic U/S, is best suited for distal biliary cancers and plastic stents may be left in place to relieve the obstruction (3).
Preoperative histological confirmation of a bile duct cancer might be difficult to obtain. Percutaneous needle biopsies and endoscopic brush biopsies have sensitivity of less than 50% and one can not rely on negative results (18). Histological confirmation is, thus, not mandatory before operative exploration. Differential diagnosis includes gallbladder cancer, metastatic disease, rare primary neoplasms (eg carcinoid), Mirrizzi´s syndrome, focal biliary sclerosis and other benign conditions. In the absence of clear evidence of unresectability, all suspected cholangiocarcinomas should be considered for resection (19).
At preoperative investigation, several criteria for unresectability have been established, which include:
– medical comorbidities limiting the patient´s ability to undergo major surgery,
– significant underlying liver disease prohibiting liver resection necessary for curative surgery,
– bilateral or multifocal intrahepatic disease on cholangiography that precludes resection,
– tumor extension to secondary biliary radicals (Bismuth´s types III, IV),
– tumor invasion of the main portal trunk,
– bilateral involvement of hepatic arterial or portal venous structures,
– lobar atrophy with contralateral portal vein involvement,
– contralateral tumor extension to secondary biliary radicals,
– evidence of metastases to N2 level lymph nodes (peripancreatic, paraduodenal, peripertal,
– celiac, superior mesenteric, posterior pancreaticoduodenal),
– presence of distant metastases.
Selection of patients who are "fit” to undergo radical surgery is sometimes different. Assessing liver function relies on standard laboratory tests of hepatic metabolic and synthetic function. B and C patients are not candidates for curative surgery if a liver resection is required. Cardiopulmonary evaluation includes pulmonary function tests (most important in smokers) and an EKG. A more invasive cardiac evaluation is indicated on patients with significant comorbidities or poor performance medical status.
STAGING, CLASSIFICATION AND PROGNOSTIC FACTORS
Multiple staging systems have been proposed. The two most commonly used are the TNM system devised by the AJCC and the modified Bismuth-Corlette classification for HCCA (20, 21), (Tables 1, 2, Figure 1). However, in both systems potential hepatic artery or portal venous involvement by the tumor and the functional status of the liver are not taken into account. Actually, according to Memorial Sloan Kettering Hospital´s experience, portal vein involvement with tumor was the only independent predictor of respectability (22). As a result, Blumgart et al. proposed a modified T-staging system, which takes into consideration both vascular involvement by tumor extension and the presence of liver atrophy (22), (Table 3).
Table 1. AJCC staging systems for cholangiocarcinoma.
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T1,2 | N0 | M0 |
| Stage IVA | T3 | Any | M0 |
| Stage IVB | Any | Any | M1 |
AJCC, American Joint Committee on Cancer; Tis, carcinoma in situ; T1, tumor invades subepithelial connective tissue; T3, tumor invades adjacent structures, i.e., liver, pancreas, duodenum, stomach, gallbladder, colon, or stomach; N1, metastasis in the cystic duct, pericholedochal, and/or hilar lumph nodes; N2, metastasis in the peripancreatic (head only), paraduodenal, periportal, celiac, superior mesenteric, and/or posterior pancreaticoduedenal lymph nodes; M0, no distant metastases.
Table 2. Modified Bismuth-Corlette classification for hilar cholangiocarcinoma.
| Type I | Below the confluence |
| Type II | Confined to confluence |
| Type IIIa | Extension into right hepatic duct |
| Type IIIb | Extension into left hepatic duct |
| Type IV | Extension into right and left hepatic ducts |
Other modifications of the Bismuth-Corlette classification system have been suggested but have not been widely accepted.

Fig. 1. Bismuth, Nakache and Diamond classification, for HCCA.
Table 3. Proposed modified T-stage criteria for hilar cholangiocarcinoma.
| T1 | Tumor confined to the confluence and/or right or left hepatic duct without portal vein involvement or liver atrophy |
| T2 | Tumor confined to the confluence and/or right or left hepatic duct with ipsilateral liver atrophy; no portal vein involvement demonstrated |
| T3 | Tumor confined to the confluence and/or right or left hepatic duct with ipsilateral portal vein branch involvement with/without associated ipsilateral lobar liver atrophy; no main portal vein involvement (occlusion, invasion, or encasement) |
| T4 | Any of the following:
1) Tumor involving both right and left hepatic ducts up to secondary biliary radicals bilaterally
2) Main portal vein encasement |
Additional prognostic factors that have been reported include histological variant of the tumor, presence of a blood vessel, lymphatic and perineural invasion, presence of lymph node involvement, and involvement of tumor margins. Data received from the American National Cancer Institute, referring on 1896 cases of extrahepatic bile duct cancers, showed that 28% had localized disease (AJCC stage, I and II) and were potential surgical candidates (2). The majority (72%) presented with either lymph node (46%) or distant (24%) metastases (AJCC stage, III and IV) (2). These results are similar to the experience reported by MSKCC (22) (Table 4).
Table 4. Factors precluding curative resection in 60 patients with hilar cholangiocarcinoma (MSKCC, 1991-1997).
| Group | None of patients (%) | Local extension (%) | Nodal metaseases (%) | Distant metastases (%) | Other (%) |
| Total evaluated | 90 | | | | |
| Unresectable based on preoperative imaging | 21 (23) | 9 (42) | 0 (0) | 8 (38) | 4 (19) |
| Unresectable at laparotomy | 39 (43) | 8 (21) | 14 (36) | 9 (23) | 8 (21) |
| Total unresectable | 60 (66) | | | | |
| Potential curative resections (preoperative) | 69 (77) | | | | |
| Total resected | 33 (48) | | | | |
| Curative resections | 30 (43) | | | | |
| Palliative resections | 3 (4) | | | | |
| Negative margin | 25 (83) | | | | |
| Positive margin | 5 (17) | | | | |
MSKCC, Memorial Sloan-Kettering Cancer Center. Preoperative factors that limited attempts at resection were related to pre-existing patient comorbidities.
Intraoperative factors limiting attempted resection were primarily related to findings of cirrhosis, additional abdominal pathology, or extensive fibrosis.
SURGICAL TREATMENT
A. Objectives, surgical approach
The primary objectives of surgical management are: a.complete tumor excision with negative histological margins, b.relief of symptoms relating to biliary obstruction, and c. restoration of bilioenteric continuity.
Before formal laparotomy, staging laparoscopy can be performed to identify lymphatic or occult peritoneal metastases not imaged by preoperative studies (3). Laparoscopic U/S can also provide accurate contact imaging of the involved biliary tract and adjacent anatomy (3). Surgical resection with negative margins is the only chance for cure (Figure 2).
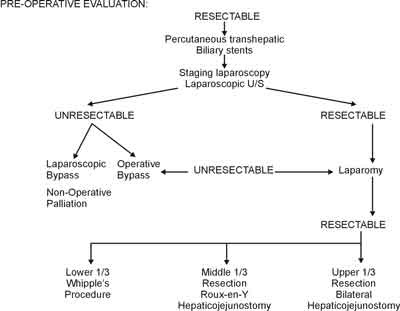
Fig. 2. Pre-operative suggested algorithm aiming to surgical resection.
Preoperative placement of percutaneous transhepatic catheters (PTCs) is part of standard management according to most authorities (3). Aggressive management of infection and correction of metabolic and coagulation disorders are also necessary. Following laparoscopic staging, exploration is undertaken via an extended right subcostal or upper midline incision.
The entire abdomen is examined for tumor spread, the liver parenchyma is carefully palpated and imaged with intraoperative U/S.
Pancreaticoduodenectomy (Whipple´s procedure) is carried out for distal third bile duct tumors and ampullary cholangiocarcinomas.
Middle third tumors are removed by resecting the extrahepatic biliary tree from above the duodenum to the base of the liver along with a lymphadenectomy of the porta hepatis. An end-to-side hepaticojejunectomy to a Roux-Y jejural loop is constructed to establish biliary-enteric continuity. The afferent limb should be at least 60 cm to prevent reflux cholangitis, which may result to anastomotic stricture.
The surgical management of hilar tumors (HCCA, Klatskin tumors) initially involves the dissection of extrahepatic biliary structures (Figure 3). Dissection of extrahepatic biliary tree from underlying vascular structures is facilitated by the early mobilization of gallbladder and division of supraduodenal common bile duct, as well as encircling left and right hepatic ducts with vessel loops (Figure 3). Preoperative placement of catheters (PTCs) facilitate operative dissection at this stage as well. The PTCs are replaced by Silastic catheters. The latter are placed over and – to distal ends of PTCs. As PTCs are pulled out of liver surface, Silastic catheters are guided into correct position (Figure 4). Bilateral hepaticojejunectomies with Silastic tube in place are constructed. Sutures of posterior row are first placed and tied. Silastic catheters are placed through jejural openings into Roux limb. Afterwards, sutures of anterior row are placed (Figure 5). This way the reconstruction is completed after resection of HCC (Figure 6).

Fig. 3. Schematic presentation indicating the dissection of the extrahepatic biliary tree facilitated by early mobilization of the gallbladder and division of supraduodenal CBO as well as encircling left and right hepatic ducts with vessel loops.
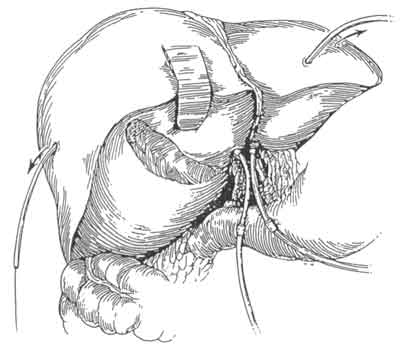
Fig. 4. The PTCs are pulled out of the liver surface and silastic catheters are guided into correct position.

Fig. 5. In this picture, sutures of the posterior row are placed and followed with the ones in the anterior row.
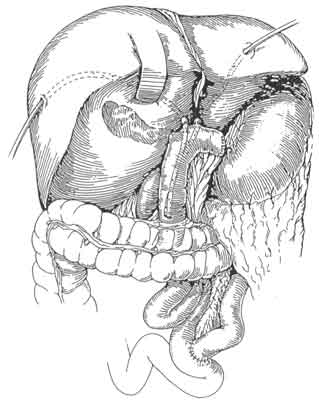
Fig. 6. The jejunal loop is in place, the reconstruction is completed after the resection of HCC, having secured the silastic tubes inside the lumen of the jejunum.
Apart from this "traditional” approach, most specialists have become more aggressive, including hepatic resection for those tumors. Following a quick assessment of whether one side of the liver is uninvolved, they resect all but the uninvolved portion of the liver. Cavitron ultrasonic aspirator (CUSA) can be useful in resection of liver parenchyma. Hepaticojejunostomy is performed well away from neoplasm. Placement of titanium clips in the resection area is necessary to help targeting postoperative external beam radiation therapy.
Occasionally, even more extensive procedures may be necessary, including back table ex vivo dissection. Total hepatectomy with orthotopic liver transplantation is an option.
B. Clinical Outcomes
Table 5 depicts selected surgical series of cholangiocarcinoma patients with respective clinical outcomes. Most groups are now routinely performing liver resections for HCCA in 50% to 100% of cases. When this approach is used, a negative histological margin is achieved in more than 50% of cases, and is associated with a significant increase or trend towards prolonged survival in all studies.
Table 5. Collective series of surgical resection for hilar cholangiocarcinoma reported since 1990.
| Author | Study years | Resected, n | Liver resection, % | Histological margin status, % | 3-year survival, % | Operative mortality (after liver resection) |
| Positive | Negative | Margin+ | Margin- |
| Hadjis9 (1990) | 8 | 27 | 60 | 44 | 56 | 17.5 moa | 22 moa | 7.4 |
| Tsuziki39 (1990) | 26 | 25 | 100 | 48 | 52 | 17 | 38 | 4 |
| Bismuth35 (1992) | 30 | 23 | 57 | 61 | 39 | 12 | 50 | 0 |
| Baer40 (1992) | 4 | 21 | 43 | 33 | 67 | 21 moa | 40 moa | 5 |
| Nagorney41 (1993) | 9 | 13 | 61 | N/A | N/A | N/A | 43 | 5 |
| Suigura42 (1994) | 18 | 83 | 100 | 43 | 57 | 11 | 41 | 8.4 |
| Su43 (1996) | 12 | 49 | 57 | 51 | 49 | 0 | 34.5 | 10.2 |
| Pichlmayr37 (1996) | 18 | 125 | 76 | 27 | 73 | 12.2 | 40.1 | 10.5 |
| Nakeeb3 (1996) | 23 | 109 | 7 | 74 | 26 | 9b | 19b | 6.7 |
| Madariaga44 (1998) | 14 | 28 | 100 | 50 | 50 | 18 | 40 | 14 |
| Figueras45 (1998) | 8 | 16 | 50 | 37 | 63 | N/A | 43 | 0 |
| Nagino46 (1998) | 19 | 138 | 90 | 22 | 78 | N/A | 42.7 | 5.6 |
| Miyazaki47 (1998) | 14 | 76 | 86 | 25 | 75 | 8 | 40 | 4.6 |
| Nimura48 (1998) | 19 | 66 | 67 | 45 | 55 | 0 | 60 | 2 |
| Burke10 (1998) | 6 | 30 | 73 | 17 | 83 | 0b | 56b | 6 |
N/A, not available.
aFigures reflect median survival.
bFigures reflect 5-year survival rates.
Nakeeb et al. reported the experience of Johns Hopkins University in managing 294 patients with HCCA during a 23-year period (4). The resectability rate increased with a more distal location (50% vs. 56% vs. 91%) and resection improved survival at each site. 5-year survival rates were 11% and 28% respectively for perihilar and distal tumors (4). These authors, though, advocated local bile duct excision only as definitive therapy for some cases of HCAA, and they performed a partial hepatectomy in only 7% of cases. This is in contrast to the experience reported by MSKCC, in which partial hepatectomy was performed in 2/3 of cases and three other reports, where hepatectomies were performed in all cases (22, 23, 24, 25). It is also noteworthy that Nakeeb et al. reported a positive histological margin 2-3 times higher than that reported in other series (26, 27). The average operative mortality rate reported by MSKCC is 6%, which compares favorably to the one from John Hopkins University (3.2%), (4, 22). Madariaga et al. performed liver resection in 100% of HCCA cases has reported the highest operative mortality (14%) (25). Evidence in support of an aggressive approach to resectional therapy for HCCA is reflected, though, in the 40% to 60%. 3-year survival rates reported in most series in which this strategy was pursued (23, 24, 25).
Another controversial approach to the management of HCCA is orthotopic liver transplantation (OLT). Reports on experience with OLT showed that resection yielded equivalent or superior overall 5-year survival at all stages of disease and radical resection itself offered the best quality of life (28). OLT should not be considered a standard form of therapy for patients with HCCA (29).
PALLIATIVE THERAPY
A. Non operative Palliation
Patients with unresectable tumors and those who are not candidates for resection should have biliary drainage tubes placed either percutaneously or endoscopically. Every attempt should be made to internalize drainage so that patients can avoid the inconvenience and morbidity of external drainage (3). This is accomplished by placement of biliary endoprostheses across the obstructing tumor, which provide drainage from above and below. Recurrent jaundice and cholangitis can result when plastic endoprostheses are occluded, dislodged or migrate. To prevent such complications, self-expanding metallic biliary endoprostheses, such as the Wallstent should be placed. Metallic stents are superior in the palliation of malignant biliary obstruction and have longer duration of patency (6-8 months) (30). Failure of jaundiceend symptoms to resolve (usually within few days) after adequate drainage usually indicates underlying lobar atrophy due to vascular compromise of the affected lobe.
B. Operative palliation
Surgical exploration is advised for good-risk patients. There are three types of palliative biliary drainage procedures: a.internal bypass without placement of indwelling tube, b.placement of indwelling tube, with multiple side holes through the obstruction, c.intestinal anastomosis to a major hepatic duct.
Unresectable lower third tumors are bypassed by end-to-side choledochojejunostomy or cholecystojejunostomy, which can be performed laparoscopically (3). It is critical to determine by cholangiography or laparoscopic U/S that the cystic duct is patent, when a cholecystojejunostomy is to be performed. Middle-third unresectable tumors can be managed by an end-to-side hepaticojejunostomy. For unresectable upper-third hilar tumors, proximal hepaticojejunostomies are performed using either the proximal segment III (most commonly) or segment V hepatic duct, with minimal operative mortality and one-year patency rate of 80% (10).
ADJUVANT THERAPY
There is no effective adjuvant therapy for cholangiocarcinoma. Intraductal brachytherapy catheters after loaded with iridium - 192 seeds are used to deliver 2000 cGy at 1em. Subsequently those patients may be treated with 5000 cGy of external beam radiation over 5 to 6 weeks (31). At present, the use of chemotherapy in the treatment of cholangiocarcinoma should be limited to clinical trials. Few small phase II chemotherapy studies have been reported, where a variety of agents (5-fluoronracil, streptojocitin, mizomycin C, methotrexate, carboplatin) have been used as single agents or in combination. Partial response rate of less than 10% only have been reported (32, 33). Finally, photodynamic therapy has been under investigation in the palliative management of extrahepatic cholangiocarcinoma (34).
Piśmiennictwo
1. Kirschbaum J.D., Kozoll D.C.: Carcinoma of the gall bladder and extrahepatic bile ducts. SGO 1941; 73:740-53. 2.Carriaga M.T., Henson D.E.: Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995; 75:171-90. 3.Callery M.P., Meyers W.C.: Bile duct Cancer In: Cameron JL. Current Surgical Therapy. 6th edition. Mosby Ine, St. Louis, Missonei, 1998; 455-61. 4.Nakeeb A., Pitt H.A., Sohn T.A., Coleman J. et al.: Cholangiocarcinoma: a spectrum of intrahepatic, perihilar, and distal tumors. Ann. Surg. 1996; 224:463-/075. 5.Tompkins R.K., Thomas D., Wile A. et al.: Prognostic factors in bile duct carcinoma. Ann. Surg. 1981; 194:447-55. 6.Reding R., Buard J.L., Lebeau G., Launois B.: Surgical management of 552 carcinomas of the bile duct (gall bladder and periampullary excluded). Ann. Surg. 1991; 213:236-41. 7.Pitt H.A., Dooley W.C., Yeo C.J., Cameron J.L.: Malignancies of the biliary tree. Curr. Prob. Surg. 1995; 32:1-90. 8.Thuluvath P.J., Rai R., Vembrux A.C., Yeo C.J.: Cholangiocarcinoma: a review. Gastroenterologist 1997; 5:306-15. 9.Blumgart L.H., Benjamin I.S.: Cancer of the bile ducts. In: Blumgart L.H., ed. Surgery of the Liver and Biliary Tract. 2nd ed. London: Churchill Livingstone, 1994; 1051-67. 10.Hochwald S.N., Burke E., Jarnigan W.R., Fong Y., Blumgart L.H.: Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch. Surg. 1999; 134:251-66. 11.Jarnigan W.R., Burke E., Power C., Fong Y., Blumgart L.H.: Intrahepatic biliary enteric bypass provides effective palliation in selected patient with malignant obstruction at the hepatic duct confluence. Am. J. Surg. 1998; 175:453-60. 12.Karsten T.M., Allema J.H., Reinders M. et al.: Preoperative biliary drainage, colonization of bile and postoperative complications in patients with tumours of the pancreatic head: a retrospective analysis of 241 consecutive patients. Eur. J. Surg. 1996; 162:881-8. 13.Heslin M.J., Brooks A.D., Hochwald S.N., Blumgart L.H., Brennan M.F.: A preoperative biliary stent is associated with increased complications after pancreaticoduodenectomy. Arch. Surg. 1998; 133:149-54. 14.Elyaderani M.K., Mc Dowell De, Zimmermann B.: Percutaneous transhepatic catheterization in reconstructive surgery of the biliary ducts. South. Med. J. 1985; 78:142-9. 15.Shaked A., Colonna J.O., Goldstein L., Busuttil R.W.: The interrelation between sclerosing cholangitis and ulcerative colitis in patients undergoing liver transplantation. Ann. Surg. 1992; 215:598-605. 16.Weinberg K., Mutumm S.S.: Pathological aspects of cholangiocarcinoma. J. Pathol. 1983; 139:217-38. 17.Skamoto E., Nimura Y., Hayakawa N. et al.: The pattern of infiltration at the proximal border of hilar bile duct cancer: a histological analysis of 62 resected cases. Ann. Surg. 1998; 227:405-11. 18.Guthrie J.A., Ward J., Robinson P.J.: Hilar cholangiocarcinomas: T2-weighted spin-echo and gadolinium-enhanced FLASH MR imaging. Radiology 1996; 201:347-51. 19.Wetter L.A., Ring E.J., Pellegrini C.A., Way L.W.: Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am. J. Surg. 1991; 161:57-62. 20.Hadjis N.S., Blenkharm J.L., Alexander N., Benjamin I.S., Blumgart L.H.: Outcome of radical surgery in hilar cholangiocarcinoma. Syrgery 1990; 107:597-604. 21.American Joint Committee on Cancer, eds. AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott-Raven, 1997. 22.Bismuth H., Nakache R., Diamond T.: Management strategies in resection for hilar cholangiocarcinoma. Ann. Surg. 1992; 215:31-8. 23.Burke E., Jarnigan W.R., Hochwald S.N., Pisters P.W.T., Fong Y., Blumgart L.H.: Hilar cholangiocarcinoma patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann. Surg. 1998; 228:385-94. 24.Tsuzuki T., Ueda M., Kuramochi S. et al.: Carcinoma of the main hepatic duct junction: indications, operative morbidity and mortality, and long-term survival. Surgery 1990; 115:495-501. 25.Suigura Y., Nakamura S., Lida S. et al.: Extensive resection of the bile ducts combined with liver resection for cancer of the main hepatic duct junction: a cooperative study of the Keio Bile Duct Cancer Study Group. Surgery 1994; 115:445-51. 26.Madariaga J.R., Iwatsuki S., Todo S., Lee R.G., Irish W., Starzl T.E.: Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann. Surg. 1998; 227:70-9. 27.Nagino M., Nimura Y., Kamiya J. et al.: Segmental liver resection for hilar cholangiocarcinoma. Hepatogastroenterology 1998; 45:7-13. 28.Miyazaki M., Ito H., Nakagawa K. et al.: Aggressive surgical approaches to hilar cholangiocarcinoma: hepatic or local resection? Surgery 1998; 123:131-6. 29.Klempnauer J., Ridder G.J., Werner M., Weimann A., Pichlmayar R.: What constitutes long-term survival after surgery for hilar cholangiocarcinoma? Cancer 1997; 79:26-34. 30.Iwatsuki S., Todo S., Marsh J.W. et al.: Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J. Am. Coll. Surg. 1998; 187:358-64. 31.Jeyarajah D.R., Klintmalm G.B.: Is liver transplantation indicated for cholangiocarcinoma? J. Hepatobil. Pancreat. Surg. 1998; 5:48-51. 32.Shapiro M.J.: Management of malignant biliary obstruction: nonoperative and palliative techniques. Oncology 1995; 9:493-6. 33.Kuvshinoff B.W., Armstrong J.G., Fong Y. et al.: Palliation of irresectable hilar cholangiocarcinoma with biliary drainage and radiotherapy. Br. J. Surg. 1995; 82:1522-55. 34.Ravry M.J., Omura G.A., Bartolucci A.A., Einhorn L., Kramer C., Davila E.: Phase II evaluation of cispatin in advanced hepatocellular carcinoma and cholangiocarcinoma: a Southeastern Cancer Study Group Trial. Cancer Treat. Res. 1986; 70:311-2. 35.Bukowski R.M., Leichman L.P., Rivkin S.E.: Phase II trial of m-AMSA in gallbladder and cholangiocarcinoma: a Southwest Oncology Group Study. Eur. J. Cancer. Clin. Oncol. 1983; 19:721-3. 36.Ortner Ma, Liebetruth J., Schreiber S. et al.: Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology 1998; 114:536-42.





