© Borgis - Postępy Nauk Medycznych 11/2010, s. 846-850
*Cezary Cybulski, Bartosz Gliniewicz, Adam Gołąb, Andrzej Sikorski, Jan Lubiński
Clinical genetics of prostate cancer
Genetyka kliniczna raka prostaty
International Hereditary Cancer Centre, Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland
Head of Department of Genetics and Pathology: prof. dr hab. med. Jan Lubiński
Streszczenie
Badania epidemiologiczne ostatniego dwudziestolecia dowodzą, że czynniki genetyczne mają ogromne znaczenie w etiologii raka gruczołu krokowego, jednakże geny związane z dziedziczną predyspozycją do tego nowotworu pozostają w dużej mierze niepoznane. Za pomocą analizy sprzężeń dotychczas zlokalizowano klika regionów chromosomalnych związanych z predyspozycją do raka gruczołu krokowego, takich jak: HPC1 (1q25-25), PCaP (1q42-43), HPCX (Xq27-28), CAPB (1p36), HPC2 (17p12), HPC20 (20q13), 8p22-23. W obrębie tych regionów zidentyfikowano trzy geny potencjalnie związane z rozwojem raka prostaty: ELAC2, RNASEL i MSR1. Jakkolwiek, żaden z nich nie jest genem wysokiej penetracji dla raka prostaty. Wiele badań opisuje zwiększone ryzyko raka stercza u nosicieli mutacji genów naprawy DNA, a zwłaszcza mutacji genu BRCA2. W Polsce prowadzimy badania, których celem jest wyjaśnienie podłoża genetycznego raka prostaty. Dotychczas wykryliśmy związek pomiędzy nosicielstwem mutacji konstytucyjnych genów BRCA1, CHEK2i NBS1a zwiększonym ryzykiem zachorowania na raka prostaty. Nasze badania dowodzą, że do poznanych genetycznych markerów wysokiego ryzyka raka prostaty również można zaliczyć nosicielstwo specyficznych zmian genów BRCA1, CHEK2 i NBS1 u mężczyzn, u których w rodzinie stwierdzono co najmniej jedno zachorowanie na raka prostaty u krewnego I lub II stopnia (ryzyko zachorowania zwiększone około 5-15-krotnie). Identyfikacja genetycznych markerów podatności na raka prostaty ma na celu usprawnienie profilaktyki, diagnostyki i postępowania z rakiem stercza w Polsce. W ostatnim roku opublikowano wyniki badań asocjacyjnych z wykorzystaniem polimorfizmów pokrywających cały genom (Genome-wide Association Studies; GWAS) dla kilku częstych nowotworów. Badania te doprowadziły do wykrycia szeregu markerów i regionów chromosomalnych związanych z predyspozycją do nowotworów. Markery podatności na raka prostaty zidentyfikowano na chromosomach 2, 3, 6, 7, 8, 10, 11, 17, 19 i X. Wykryte markery mogą wejść w skład panelu zbiorczego markerów określających grupy ryzyka dla raka stercza.
Summary
Epidemiologic research conducted over the last two decades has led us to believe that inherited factors play an important role in the etiology of prostate cancer, but the genes, which underlie the inherited susceptibility are elusive. Through linkage analysis, numerous prostate cancer susceptibility chromosomal loci have been identified including HPC1 (1q25-25), PCaP (1q42-43), HPCX (Xq27-28), CAPB (1p36), HPC2 (17p12), HPC20 (20q13) and 8p22-23. Three candidate susceptibility genes have been positionally cloned. HPC1, HPC2/ELAC2, and MSR1, but any of these is not a high-risk prostate cancer susceptibility gene. The most compelling associations for prostate cancer described to date are with genes involved in the DNA damage repair including, including BRCA2. In Poland, we have initiated a program to identify DNA variants, which confer an increased risk of prostate cancer. We found that germline mutations in BRCA1, CHEK2, NBS1confer an increased prostate cancer risk in Polish men. Our studies provide evidence that the list of known genetic markers of high risk of prostate cancer can be extended by specific mutations in NBS1, BRCA1 and CHEK2 genes in men with a positive family history of prostate cancer in at least one first or second degree relative (the risk increased about 5-15 fold). Identification of genetic markers of prostate cancer susceptibility will improve prevention, diagnosis and management with prostate cancer in Poland. In the past year, the results of several genome-wide searches for loci for cancer susceptibility prostate cancer have been reported. Several chromosomal regions of interest have been identified, including loci on chromosomes 2, 3, 6, 7, 8, 10, 11, 17, 19 and X. Identification of genetic markers of prostate cancer susceptibility will improve prevention, diagnosis and management with prostate cancer. These DNA variants may be included in the panel of markers designed to estimate the risk of prostate cancer in a given population.

Familial clustering of prostate cancer was first described in 1955, and a term hereditary prostate cancer was first used in 1992 by Carter who reported the results of linkage analysis in a series of 691 men with prostate cancer (PC) (1). This analysis revealed that 9% cases of familial clustering of prostate cancer is associated with a single rare allele. Penetrance of this allele was 88% to age of 85. The allele conferring high risk for prostate cancer was found on long arm of chromosome 1 (1q24-25), and this locus was named HPC1 (2). Other prostate cancer susceptibility chromosomal loci have been identified using linkage analysis [i.e. PCaP (1q42-43), HPCX (Xq27-28), CAPB (1p36), HPC2 (17p12), HPC20 (20q13) and 8p22-23]. From these regions three candidate susceptibility genes have been positionally cloned -RNASEL, HPC2/ELAC2, and MSR1, but any of these is not a high-risk prostate cancer susceptibility gene.
Epidemiologic research conducted over the last two decades has led us to believe that inherited factors play an important role in the etiology of prostate cancer. Familial clustering of prostate cancer is observed in about 10-20% of cases. Genes of high penetrance may be responsible for 5-10% of prostate cancer cases and for as many as 30-40% of early onset prostate cancer (3). Scandinavian study of twins suggested that the heritability of prostate cancer may be as high as 42% (4).
FAMILY HISTORY AND PROSTATE CANCER RISK
Familial clustering of prostate cancer is important risk factor for prostate cancer (3, 5). The risk of prostate cancer by the presence of prostate cancer in first and second degree relatives is shown in table 1.
Table 1. Family history and prostate cancer risk.
| Family history | Relative Risk |
| Negative | 1 |
| Father with PC at age of 60 or above. | 1.5 |
| 1 brother with PC at age of 60 or above. | 2 |
| Father with PC before age of 60 | 2.5 |
| 1 brother with PC before age of 60 | 3 |
| 2 first degree relatives with PC | 4 |
| 3 or more relatives with PC | 5 |
Clinical criteria for HPC (Carter; fig. 1a, 1b) (6).
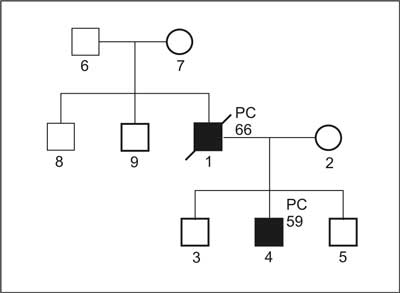
Fig. 1a. Pedigree of family with diagnosis of HPC.
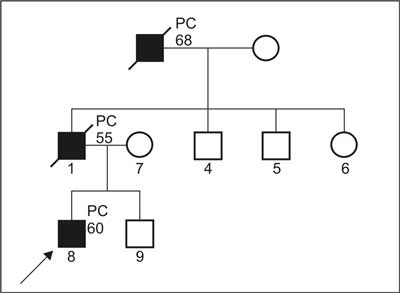
Fig. 1b. Pedigree of family suspected for HPC, which do not fullfil clinical criteria.
1. Definitive diagnosis of HPC:
a) PC in 3 or more first degree relatives; or
b) PC in 3 generations; or
c) PC at age below 56, in two or more relatives.
2. Diagnosis of cases suspected for HPC:
a) PC in 3 or more relatives but not matching point a) or b) for definitive diagnosis; or
b) PC in 2 relatives, including at least one diagnosed below age of 60 and/or vertical transmission but without fulfilling point c) for definitive diagnosis; or
c) at least one PC below age of 50, not matching criteria for definitive diagnosis.
Clinical characteristics of HPC
The most important features of HPC include: autosomal dominant inheritance of prostate cancer (rarely autosomal recessive inheritance or X-linked dominant inheritance), and early age of diagnosis – the mean age below 56, so 6-7 years younger than that seen in sporadic cases (2). Because of early onset of disease, prostate cancer is more common cause of death in HPC cases (75%), then in sporadic cases (50%) (7, 8).
Other cancers in families with HPC
Epidemiological studies provide evidence that in families with prostate cancer the risk of prostate cancer only is increased. Some studies suggested increased risk for brain tumors, stomach cancer or breast cancer in HPC families, but in most studies HPC is characterized as susceptibility to prostate cancer only – site specific hereditary prostate cancer (9).
HEREDITARY CANCER SYNDROMS AND PC RISK
BRCA1 mutations confer high risk of breast and ovarian cancer. Several studies suggested two – fold increased risk of prostate cancer in Ashkenazi Jewish men with a BRCA1 mutation (185delAG or 5382insC). Other studies, in non-Jewish populations, have found little or no evidence of an increased risk for prostate cancer in BRCA1 carriers (10-16).
Association of BRCA2 mutations with increased prostate cancer risk is well documented. It was reported that carriers of germline mutations in BRCA2 are at 5-fold increased risk of PC, the risk is higher (increased by 7-fold) to age of 65, and even 20-fold to age of 56 (9). Recent studies suggest that BRCA2 carriers develop aggressive prostate cancer, with high grade (G3, G4), and of early onset (5 years younger on average than non-carriers). Prognosis was reported to be much worse for men with PC and with a BRCA2 mutation, i.e. in one study the mean survival was 2 years in BRCA2 carriers versus 12 years in non-carriers. Mutations in BRCA2or BRCA1are rare and the contribution of these two genes to prostate cancer etiology is relatively small.
In addition increased prevalence of prostate cancer was observed in families with Cowden syndrome, Li-Fraumeni syndrome, and hereditary stomach cancer caused by mutations of E-cadherin (3, 9).
CANDIDATE GENES FOR HPC
Through linkage analysis, numerous prostate cancer susceptibility chromosomal loci have been identified [i.e. PCaP (1q42-43), HPCX (Xq27-28), CAPB (1p36), HPC2 (17p12), HPC20 (20q13) and 8p22-23]. From these regions three candidate susceptibility genes have been positionally cloned -RNASEL, HPC2/ELAC2, and MSR1. RNASEL gene was cloned within HPC1 locus. RNASEL is an endoribonuclease involved in the mediation of the antiviral and proapoptotic activities. Two segregating mutations (Glu265X and Met1Ile) were found in the RNASEL gene. In addition, it has been reported that other RNASEL variants (471delAAAG, Arg462Gln) may confer prostate cancer risk. ELAC2 gene was identified in HPC2 locus. Its product was shown to possess tRNase activity and to interact with gamma-tubulin, a component of the mitotic apparatus, suggesting a possible role for ELAC2 in the regulation of cell cycle progression. So far, segregating mutations in ELAC2have been found in three prostate cancer families. In addition, several polymorphisms have been identified, some of which (Ser217Leu i Ala541Thr) may be associated with an elevated risk for prostate cancer. The latest identified prostate cancer-susceptibility gene is the macrophage scavenger receptor 1 (MSR1) gene, located at 8p22-23. The MSR1 protein, a scavenger receptor, has been linked to a wide variety of normal and pathological processes, including inflammation, innate and adaptive immunity, oxidative stress, and apoptosis. Segregating germ-line mutations (including truncating mutation – Arg193X) in MSR1 have been reported in several families affected with hereditary prostate cancer. In addition, a common missense MSR1 variants associated with an increased risk for prostate cancer have been reported (17, 18, 19). Unfortunately further studies, also our studies in Polish men, provided evidence that none of the genes found for HPC in is a true high-risk susceptibility gene for prostate cancer (20).
There is evidence that rare mutations of genes in the DNA damage signaling pathway and cell cycle control pathway ( CHEK2 and NBS1) confer increased PC risk. Common variants in the genes in these pathways ( CDKN1B, CDKN1A, ATM, XRCC1, ERCC2) also have been associated with prostate cancer, however based on single studies (21, 22, 23).
CHEK2 [CHEK2, also known as "CHK2” (MIM 604373)] is located on chromosome 22q and encodes the human analog of yeast Cds1 and Rad53, which are checkpoint kinases. Activation of these proteins in response to DNA damage prevents cellular entry into mitosis.
Mutations in the CHEK2have been found to be associated with prostate cancer risk in the United States and Finland (24, 25). In a study from the United States, 18 different CHEK2mutations were found, mostly in single patients (24). In Finland two variants of CHEK2 (1100delC, I157T) were associated with prostate cancer risk (25). Truncating mutations of CHEK2 increase prostate cancer risk by 2-3 fold. However, the risk of prostate cancer for man with a CHEK2 mutation is not determined solely by the presence of the mutation; penetrance is also dependent on the family history of cancer. That is, the risk for man with a mutation and a positive family history of prostate cancer is greater than that of a carrier of the same mutation who has no family history of prostate cancer. For example the risk for prostate cancer associated with CHEK2 1100delC mutation in Finnish population in familial cases was increased 8-fold.
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Carter BS, Beaty TH, Steinberg GD et al.: Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA 1992; 89: 3367-71.
2. Smith JR, Freije D, Carpten JD et al.: Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 1996; 274: 1371-4.
3. Bratt O: Hereditary prostate cancer: clinical aspects. J Urol 2002; 168: 906-13.
4. Lichtenstein P, Holm NV, Verkasalo PK et al.: Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343: 78-85.
5. Virtanen A, Gomari M, Kranse R et al.: Estimation of prostate cancer probability by logistic regression: free and total prostate-specific antigen, digital rectal examination, and heredity are significant variables. Clin Chem 1999; 45: 987-94.
6. Carter BS, Bova GS, Beaty TH et al.: Hereditary prostate cancer: epidemiologic and clinical features. J Urol 1993; 150: 797-802.
7. Bratt O, Damber JE, Emanuelsson M et al.: Hereditary prostate cancer: clinical characteristics and survival. J Urol 2002; 167: 2423-6.
8. Keetch DW, Humphrey PA, Smith DS et al.: Clinical and pathological features of hereditary prostate cancer. J Urol 1996; 155: 1841-3.
9. Sigurdsson S, Thorlacius S, Tomasson J et al.: BRCA2 mutation in Icelandic prostate cancer patients. J Mol Med 1997; 75: 758-61.
10. Struewing JP, Hartge P, Wacholder S et al.: The risk of cancer associated with specific mutations of BRCA1and BRCA2 among Ashkenazi Jews. N Engl J Med 1997; 336: 1401-8.
11. Warner E, Foulkes W, Goodwin P et al.: Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 1999; 91: 1241-7.
12. Giusti RM, Rutter JL, Duray PH et al.: A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J Med Genet 2003; 40: 787-92.
13. Thompson D, Easton DF: Breast Cancer Linkage Consortium: Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 2002; 94: 1358-65.
14. Sinclair CS, Berry R, Schaid D et al.: BRCA1 and BRCA2 have a limited role in familial prostate cancer. Cancer Res 2000; 60: 1371-5.
15. Ikonen T, Matikainen MP, Syrjäkoski K et al.: BRCA1 and BRCA2 mutations have no major role in predisposition to prostate cancer in Finland. J Med Genet 2003; 40: E98.
16. Zuhlke KA, Madeoy JJ, Beebe-Dimmer J et al.: Truncating BRCA1 mutations are uncommon in a cohort of hereditary prostate cancer families with evidence of linkage to 17q markers. Clin Cancer Res 2004; 10: 5975-80.
17. Carpten J, Nupponen N, Isaacs S et al.: Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet 2002; 30: 181-4.
18. Casey G, Neville PJ, Plummer SJ et al.: RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet 2002; 32: 581-3.
19. Xu J, Zheng SL, Komiya A et al.: Germline mutations and sequence variants of the macrophage scavenger receptor 1 gene are associated with prostate cancer risk. Nat Genet 2002; 32: 321-5.
20. Cybulski C, Wokołorczyk D, Jakubowska A et al.: DNA variation in MSR1, RNASEL and e-cadherin genes and prostate cancer in Poland. Urol Int 2007; 79: 44-9.
21. Kibel AS, Suarez BK, Belani J et al.: CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer Res 2003; 63: 2033-6.
22. Angčle S, Falconer A, Edwards SM et al.: ATM polymorphisms as risk factors for prostate cancer development. Br J Cancer 2004; 91: 783-7.
23. Rybicki BA, Conti DV, Moreira A et al.: DNA repair gene XRCC1 and XPD polymorphisms and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev 2004; 13: 23-9.
24. Dong X, Wang L, Taniguchi K et al.: Mutations in CHEK2 associated with prostate cancer risk. Am J Hum Genet 2003; 72: 270-80.
25. Seppälä EH, Ikonen T, Mononen N et al.: CHEK2 variants associate with hereditary prostate cancer. Br J Cancer 2003; 89: 1966-70.
26. Easton DF, Eeles RA: Genome-wide association studies in cancer. Hum Mol Genet 2008 Oct 15; 17 (R2): R109-15.
27. Takata R, Akamatsu S, Kubo M et al.: Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet 2010 Sep; 42 (9): 751-4.
28. Cybulski C, Górski B, Debniak T et al.: NBS1 is a prostate cancer susceptibility gene. Cancer Res 2004; 64: 1215-9.
29. Cybulski C, Górski B, Gronwald J et al.: BRCA1 mutations and prostate cancer in Poland. Eur J Cancer Prev 2007 (in press).
30. Cybulski C, Huzarski T, Górski B et al.: A novel founder CHEK2 mutation is associated with increased prostate cancer risk. Cancer Res 2004; 64: 2677-9.
31. Cybulski C, Wokołorczyk D, Huzarski T et al.: A large germline deletion in the CHEK2 kinase gene is associated with an increased risk of prostate cancer. J Med Genet 2006; 43: 863-6.
32. von Eschenbach A, Ho R, Murphy GP et al.: American Cancer Society guidelines for the early detection of prostate cancer: update, Cancer 1997; 80: 1805-7.
33. Wolf AM, Wender RC, Etzioni RB et al.: American Cancer Society Prostate Cancer Advisory Committee. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin 2010 Mar-Apr; 60 (2): 70-98.
34. Machoy P, Lubiński J: Dziedziczny rak prostaty. Urologia Polska 2002; 55: 3.


