© Borgis - New Medicine 4/2010, s. 152-155
*Małgorzata Tokarska-Rodak1, Justyna Niedźwiadek1, Hanna Fota-Markowska2, Filip Śmiechowicz2, Bożena Gajownik3, Roma Modrzewska2, Maria Kozioł-Montewka1
Antinuclear antibodies in patients with Lyme disease
1Department of Medical Microbiology, Medical University, Lublin, Poland
Head of Department of Medical Microbiology: prof. dr hab. Maria Kozioł-Montewka
2Department of Infectious Diseases, Medical University, Lublin, Poland
Head of Department of Infectious Diseases: prof. dr hab. n. med. Roma Modrzewska
3Clinic of Infectious Diseases, Public Hospital in Łuków, Poland
Head of Clinic of Infectious Diseases: Bożena Gajownik
Summary
Aim. The aim of the study was to determine the relation between B. burgdorferi infection in patients with clinical symptoms of Lyme disease and presence of antinuclear antibodies (ANA) depending on the duration of the disease and in the aspect of the erythema migrans (EM) experience.
Material and methods. The study was conducted in a group of 110 patients with the second stage of Lyme disease hospitalized during 2007-2009. The ANA were determined by the IIFT test and the Western blot test.
Results. Positive ANA results (IIFT test) were obtained for 16 patients. The presence of ANA (Western blot test) was confirmed in 4 women who reported a single bite by ticks before 2000, EM presence in the past and the administration of antibiotic treatment due to Lyme disease because of EM and ailments concerning bone-joint structure but without the presence of autoimmune diseases.
Conclusions. The relation between primary diagnosis of Lyme disease in the form of EM and occurrence in the late period is possible despite the application of treatment of joint symptoms with simultaneous presence of ANA antibodies.
Introduction
The primary cause of Lyme disease is always infection with spirochetes of B. burgdorferi s.l. However, the clinical course of the disease is to some extent dependent on the bacteria gene-species, their number and effectiveness of the immune system of the infected person. B. burgdorferi can survive through unfavourable periods and avoid the attack from the immunological system of a host by using the strategy of creating atypical forms
(1, 2). In the specific conditions of an environmental stress, the spirochetes can undergo reversible transformation from the motile helical forms into non-motile spherical cysts. These forms have been observed in the cerebrospinal fluid and in tissues of patients with Lyme disease (3). The metabolically inactive forms ?blebs?, which contain genetic material of B. burgdorferi, are also a source of long-term antigenic stimulation. It is possible that the presence of atypical forms is also a cause of converted symptoms of the disease and the necessity of repeating the antibiotic therapy (4, 5, 6).
In spite of the enormous knowledge concerning the clinical process of B. burgdorferi infection and the development of therapeutic schemas, it is difficult to unambiguously determine the mutual host-pathogen relations which determine the effective and complete elimination of the spirochetes.
Despite the application of antibiotic therapy in the erythema migrans phase, the transition of Lyme disease into disseminated and late stage is infrequently avoided (7, 8, 9, 10). The cognition and comprehension of those numerous relations and mechanisms of their formation can have an influence on the improvement of applied methods, prevention and effective treatment of B. burgdorferi infection.
Aim of the study
The aim of the study was to determine the relation between B. burgdorferi infection in patients with clinical symptoms of Lyme disease and presence of antinuclear antibodies depending on the duration of the disease and in the aspect of the erythema migrans (EM) experience.
Material and methods
The study was conducted in a group of 110 patients: 44 men (age 20-66 yrs) and 66 women (age 20-60 yrs) with the second stage of Lyme disease, hospitalized in the Clinic of Infectious Diseases, Medical University of Lublin, and the Department of Infectious Diseases, Hospital in Łuków, in 2007-2009. The study group involved patients demonstrating manifestations of joint involvement including arthralgia and/or arthritis. The diagnosis of borreliosis was established on the basis of the patient?s medical history, physical examination, clinical picture, and serological investigation (ELISA and Western blot).
All patients were asked to respond to a questionnaire to gather information about the dates and frequency of tick bites, incidents of EM, symptoms that occurred following the tick bite and antibiotics taken.
The antinuclear antibodies were determined by the IIFT test among all of the examined patients (Biomedical Diagnostics). In the patients where a titre of 1:200 or more was obtained, the Western blot test (Euroimmun) was carried out on the quality indication in vitro with human IgG antibodies against 15 antigens: nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, ribosome protein P and AMA-M2.
Results
The patients examined in 2007-2009 due to Lyme disease with dominant joint symptoms reported in the completed survey about the time (year) of being bitten by ticks, the multiple of a bite and the application of antibiotic therapy in the past due to clinical symptoms appearing after tick bites (fig. 1).
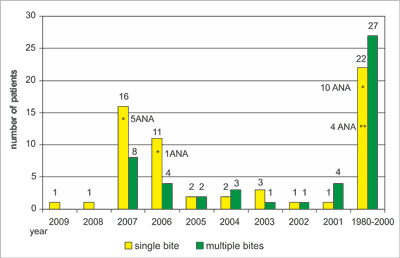
Fig. 1. Year of the tick bites reported by patients with clinical symptoms of Lyme disease and patients with positive result of ANA test.
*positive result of ANA test (IIFT)
**positive result of ANA test (Wb)
The early stage of located Lyme disease (EM) appeared among 65 from 110 examined patients (59%) in the past and for that reason 43 patients from this group (66%) underwent antibiotic treatment. Twenty-two patients (34%) in spite of the presence of EM did not undergo treatment. Among all of these patients, regardless of the treatment?s application or the lack of it, the clinical symptoms of the second stage of Lyme disease were developed.
EM did not appear in 45 patients (41%), while the symptoms of early disseminated Lyme disease appeared among all of the patients from this group. Lyme disease was diagnosed among subsequent 22 patients (49%) in 2007-2009 (i.e. during the present research).
All of the examined patients reported simultaneous appearance of various clinical ailments with Lyme disease. Ailments concerning bone-joint structure were noted frequently by 105 patients (95%), among which 12 cases concerned arthritis (13%).
Sixty patients (54%) reported muscle pains, 29 (26%) reported headaches, and 24 (22%) reported concentration disorder. Among 110 patients, 21 (19%) reported the presence of other symptoms such as meningitis, sensory loss, stiffening and oedema of the lower limbs, skin changes different from EM, fever, tinnitus and impaired sharpness of vision.
The determination of the ANA was implemented by the IIFT method in 110 patients with Lyme disease (fig. 2). Positive results of the test were obtained in 16 patients. The titre of 1:200 or more was accepted as a positive result. Cytoplasmic fluorescence was observed among
12 cases, with 4 different types of fluorescence: mitochondrial, granular nucleus, homogeneous nucleus.

Fig. 2. Medical history findings in 16 patients with Lyme arthritis and positive result of ANA tests.
*single tick bite, **double tick bite, EM ? presence of EM, O treatment, ? no treatment
In the patients with a positive result of the IIFT test, the Western blot test was carried out on the quality indication in vitro with human IgG antibodies against 15 antigens: nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, ribosome protein P and AMA-M2.
The following results were obtained:
? 1 patient (mitochondrial type of fluorescence) ? IgG anti-AMA-M2, Ro 52, SSB,
? 1 patient (type of fluorescence granular nucleus)
? IgG anti-CENP B
? 1 patient (type of fluorescence granular nucleus)
? IgG anti-SSA
? 1 patient (type of fluorescence homogeneous nucleus) ? IgG anti-nucleosomes.
Discussion
B. burgdorferi s.l. infection can manifest in the form of EM within a few days from the moment of the spirochetes? penetration into the human body. The appearance of EM is clear evidence of infection, but it does not appear in over 50% of patients bitten by infected ticks. The skin changes can be accompanied by flu-like symptoms such as malaise, fatigue, headache, defect and regional lymphadenopathy (3, 11).
From the 110-person group with Lyme disease with predominant bone-joint symptoms, EM appeared in the past in 59% of examined patients. Sixty-six percent of patients underwent antibiotic treatment in accordance with established therapeutic treatment standards of localized Lyme disease due to EM presence. Even though the disease was recognized in the early stage, the treatment did not eliminate the B. burgdorferi infection. A worrying fact is that no less than 34% of patients who had EM did not recognize it as a symptom of a disease or infection. Forty-four percent of patients from the examined group were bitten by the ticks in 1980-2000, which suggests the possibility of long-lasting infection with B. burgdorferi and thus long-lasting contact of a host?s body with anti-gene proteins of ticks. The researchers name long-lasting persistence of the disease as one of the risk factors of the occurrence of Lyme disease resistant to treatment. It has been estimated that in about 10% of patients the disease can persist for a long time and mild symptoms can remain for 6 months after the end of treatment despite repeated antibiotic therapy.
It is possible that long-lasting infection of B. burgdorferi can also induce auto-immunological changes in a small percentage of patients (6, 12). According to Singh, one potential explanation for antibiotic-resistant Lyme disease is the generation of autoimmunity mediated directly or indirectly by the pathogen (3).
The metabolically inactive vesicular forms of Borrelia (blebs) containing lipoproteins OspA, OspB, OspD are the source of a long-term antigenic stimulation which can even persist during the absence of bacteria capable of multiplication (13, 14, 15). The cross-reaction with bacterial epitope OspA163-175 is possible, although the auto-antigen participating in the reaction remains unknown. Impairment of lymphocytes? and other leukocytes? apoptosis is associated with the risk of autoimmunization on account of the participation of this process in the control and physiological extinguishing of the inflammatory and immunological response in the course of Lyme disease (5).
Over the years, the relation between clinical diseases which were associated in varying degrees with B. burgdorferi infection and the presence of antinuclear antibodies was researched. The research concerned, as well as skin changes typical for Lyme disease such as EM, circumscribed sclerosis and rheumatic changes (16).
The research carried out by Śpiewak in patients with early Lyme disease did not reveal a direct connection between the presence of anti-Borrelia antibodies and ANA (14).
The presence of IgG antinuclear antibodies was confirmed in 4 women who reported a single tick bite before 2000, EM presence in the past and the administration of antibiotic treatment due to Lyme disease because of EM and ailments concerning bone-joint structure but without the presence of autoimmune diseases.
The clinical diversity of the course of Lyme disease is relevant for the effectiveness of the immunological system of an infected person. The presence of miscellaneous factors supporting and intensifying the immunological response in tissues which are occupied with the pathological process results in the occurrence of inflammatory infiltration regardless of the presence of the active spirochetes. Difficulties are possible in the regulation of the inflammatory response and its termination after the activation period caused by an infection and in some cases also in the development of the autoimmune reaction (2, 3).
It is possible that the autoimmune processes can contribute to this excessive and inadequate inflammatory response in late-stage Lyme disease and can be responsible for sustaining inflammatory reactions after the elimination of the pathogen.
Conclusions
1. The long-lasting persistence of the disease and thus long-term antigenic stimulation can be considered as a factor enabling the initiation of autoimmune reactions. This process can exist in a small percentage of patients with Lyme disease but the possibility of its inception cannot be completely negated.
2. The relation between primary diagnosis of Lyme disease in the form of EM and occurrence in the late period is possible despite the application of treatment for joint symptoms with simultaneous presence of ANA antibodies.
Piśmiennictwo
1. Sigal LH: Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol 1997; 15: 63-91.
2. Kisand KE, Pr?kk T, Kisand KV: Propensity to excessive proinflammatory response in chronic Lyme borreliosis. APMIS 2007; 115:134-41. 3. Singh SK, Girschick HJ: Lyme borreliosis: from infection to autoimmunity. Clin Microbiol Infect 2004; 10: 598-614.
4. Kondrusik M et al.: Failures of antibiotic treatment in Lyme arthritis. Przegl Epidemiol 2008; 62: 581-588. 5. Grygorczuk S et al.: Activity of the caspase-3 in the culture of peripheral blood mononuclear cells stimulated with Borrelia burgdorferi antigens. Przegl Epidemiol 2008; 62: 85-91. 6. Wilgat P et al.: Activity of lisosomal exoglycosidases in serum of patients with chronic borrelia arthritis. Przegl Epidemiol 2004; 58: 451-58. 7. Feder HM et al.: A critical appraisal of ?chronic Lyme disease?. N Engl J Med 2007; 357: 1422-30. 8. Wormser GP, Shapiro ED: Implications of gender in chronic Lyme disease. JWH 2009; 18: 831-834. 9. Steere AC, Coburg J, Glickstein L: The emergence of Lyme disease. J Clin Invest 2004; 113: 1093-101. 10. Grygorczuk S et al.: Concentration of sFAS and sFASL in the supernatant of PBMC culture from the patients with late Lyme borreliosis. Przegl Epidemiol 2007; 61: 51-58. 11. Aguero-Rosenfeld ME et al.: Diagnosis of Lyme Borreliosis. Clin Microb Rev 2005; 18: 484-509. 12. Grygorczuk S et al.: Failures of antibiotic treatment in Lyme arthritis. Przegl Epidem 2008; 62: 581-588. 13. Brorson O, Brorson SH: Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 1997; 25: 240-6. 14. Śpiewak R, Stojek NM, Chmielewska-Badora J: Antinuclear antibodies are not increased in the early phase of Borrelia infection. Ann Agric Environ Med 2004; 11: 145-148. 15. Stere AC, Falk B, Drobin EE: Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum 2003; 48: 534-40. 16. Wojas-Pelc A, Wielowieyska-Szybińska D, Kiełtyka A: Presence of the antinuclear antibodies and antibodies to Borrelia burgdorferi among patients with morphea en plaque, deep linear scleroderma and atrophoderma Pasini-Pierini. Prz Lek 2002; 59: 898-902.

