© Borgis - Postępy Nauk Medycznych 8/2012, s. 651-659
*Paweł Dąbek, Włodzimierz Hendiger, Grzegorz Madycki, Walerian Staszkiewicz
Zmiany przepływu krwi w obrębie naczyń trzewnych i miednicy u chorych po implantacji stentgraftu z powodu tętniaka aorty brzusznej
Changes in blood flow within visceral vessels and vessels of the pelvis in patients after stent graft implantation due to an abdominal aortic aneurysm
Department of Vascular Surgery and Angiology of the Medical Centre for Postgraduate Education, The Jerzy Popiełuszko Memorial Bielański Hospital
Head of Department: prof. Walerian Staszkiewicz MD, PhD
Streszczenie
Wstęp. Operacje tętniaków aorty i tętnic biodrowych wiążą się z występowaniem pooperacyjnego niedokrwienia okrężnicy i umieralnością ogólną z tego powodu 1-8%. Wewnątrznaczyniowe techniki leczenia tętniaków przy użyciu stentgraftu nie uwolniły chirurgii od tego powikłania. Posługując się badaniem USG-cdd tętnic trzewnych uzyskujemy informacje o stanie łożyska trzewnego, co pozwala określić ryzyko wystąpienia tego powikłania.
Materiał i metody. Rozpoczęto badania prospektywne USG-cdd tętnic trzewnych na 60-osobowej grupie chorych leczonych z powodu TAB stentgraftem w porównaniu do 78-osobowej grupy kontrolnej chorych leczonych odlegle z powodu TAB i zespołu Leriche operacją otwartą wszczepienia protezy aortalnodwuudowej. W obu grupach posługiwano się techniką USG-cdd i oceniano morfologicznie i czynnościowo tętnice trzewne: PT, TKG, TKD, tętnice biodrowe wewnętrzne. Wstępnie przebadano 10 chorych. Określano standardowe parametry przepływu krwi metodą dopplerowską. Pomiary wykonywano przed i po operacji wszczepienia stentgraftu. Dla określenia ryzyka niedokrwienia posługiwano się opracowanymi wcześniej tzw. wskaźnikami niedokrwienia: prędkościowym i oporowym.
Wyniki. W przebadanym materiale prognozowano przy pomocy USG-cdd przed operacją i potwierdzono klinicznie po operacji 3 łagodne przypadki niedokrwienia miednicy pod postacią chromania i 1 przypadek niedokrwienia okrężnicy pod postacią przemijającej biegunki. Opisano ultrasonograficzne czynniki ryzyka niedokrwienia i ustalono sposób określenia rodzaju niedokrwienia (okluzyjne/nieokluzyjne).
Wnioski. 1) USG-cdd tętnic trzewnych pozwala prognozować niedokrwienie okrężnicy po operacji TAB. 2) Parametrem prognostycznym niedokrwienia okluzyjnego jest wskaźnik prędkościowy niedokrwienia (WPN) znacznie niższy do jedności (<< 1,0), a pomocniczym wskaźnik oporowy niedokrwienia (WON) wyższy od jedności (≥ 1,0). 3) Parametrem prognostycznym niedokrwienia nieokluzyjnego jest wskaźnik oporowy niedokrwienia (WON) niższy od jedności (≤ 1,0), a pomocniczym wskaźnik prędkościowy niedokrwienia (WPN) wyższy od jedności (≥ 1,0). 4) Po operacji wszczepienia stentgraftu dominuje czynnik okluzyjny niedokrwienia. 5) Po operacji klasycznej TAB działają oba czynniki niedokrwienia okluzyjny i nieokluzyjny.
Summary
Introduction. Surgery of aortic aneurysms and iliac artery aneurysms are associated with postoperative ischemic colitis and corresponding general mortality of 1-8%. Endovascular aneurysm treatment technique using stent graft did not prevent the occurrence of this complication. Using CDD ultrasound for the visceral arteries evaluation, we obtain information on condition of the celiac vascular bed, which allows determining the risk of this complication occurrence.
Material and methods. A prospective study started in reference to Colour Duplex-Doppler (CDD) ultrasound evaluation of the visceral arteries in a group of 60 patients treated for AAA by a stent graft compared to a control group of 78 patients undergoing long-term treatment due to AAA and Leriche syndrome treated with open surgery of aortobifemoral Y-graft implantation. In both groups, CDD ultrasound technique was used in order to evaluate morphological and functional aspect of the visceral arteries: CT, SMA, IMA, and the internal iliac arteries. Initially, 10 patients were examined. Standard parameters of blood flow were determined by Doppler method. Measurements were performed before and after a stent graft implantation. In order to determine the risk of ischemia previously developed so-called indicators of ischemia were used: velocity and resistance.
Results. In the studied material, predictions with CDD ultrasound were made before surgery and these predictions were verified after a surgery, which resulted in clinical confirmation of three cases of mild pelvic ischemia in the form of intermittent claudication, and 1 case of ischemic colitis in the form of transient diarrhoea. The ultrasound risk factors for ischemia were described and the manner of establishing type of ischemia (occlusive/non-occlusive) was developed.
Conclusions. 1) CDD ultrasound evaluation of the visceral arteries is able to predict colonic ischemia after AAA surgery. 2) A prognostic parameter for an occlusive ischemia is the velocity ratio of ischemia (VRI), which is significantly lower than one (<< 1.0), and the secondary resistance ratio of ischemia (RRI), which is higher than one (≥ 1.0). 3) A prognostic parameter for a non-occlusive ischemia is RRI lower than one (≤ 1.0), and secondary VRI higher than one (≥ 1.0). 4) After a stent graft implantation, an occlusive factor of ischemia is dominating. 5) After the AAA surgery, both factors of ischemia are acting, an occlusive and non-occlusive.

Introduction
In recent years, classical open surgeries for implantation of an artificial bypass graft in treatment of abdominal aortic aneurysms (AAA) have been replaced with a stent graft implantation through an inguinal approach (1).
Inserting prosthesis into the aneurysmal sac-implantation of a stent graft through a small cut at the femoral artery, and then with use of new excellent equipment, through percutaneous puncture of the artery, is commonly used method of protecting the patient against severe complications or death due to the aneurysm rupture (2).
Abdominal aortic aneurysms are quite frequently accompanied by aneurysmal dilation of the iliac arteries, which also require endovascular repair (3).
Endovascular repair surgery of the abdominal aorta with a stent graft (EVAR) related to lower surgical risk, is not free from secondary interventions, also after a few years following implantation (4, 5).
EVAR allows performing the procedure in a patient, who is at high risk of classical surgery (6, 7). During the procedure, the vessels, which are the branches of the abdominal aorta (the inferior mesenteric artery – IMA, the lumbar arteries), or sometimes the branches of the common iliac arteries (internal iliac arteries – IIA), are covered with an implant (8).
Complete morphological evaluation of the trunks of the visceral arteries and iliac arteries is sufficient in CT-scan with administration of the contrast medium (CT angiogram) (9).
Morphological and functional evaluation is possible in ultrasound evaluation using colour duplex Doppler technique (CDD ultrasound) (10). Collaterals and final intestinal arteries remain beyond the range of a direct evaluation (11). Through measurement of some flow ratios (pulsation index – PI, resistance index – RI) we may establish approximate condition of the intestinal resistance bed, which includes arterioles, precapillary sphincters and capillaries (fig. 1) (12).
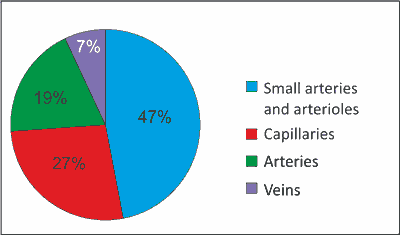
Fig. 1. Vascular components of the peripheral resistance.
Evaluation of the visceral arteries under constant technical conditions, and under fasting condition, is conducted before and after surgery (11). A question arises regarding ability to evaluate the visceral bed of the inferior segment of the alimentary tract in patients undergoing endovascular surgeries (13).
aim of the study
Detection of changes in blood flow parameters within the area of the abdominal cavity and the pelvis after stent graft implantation in treatment of the aortic aneurysm comparing to classical surgeries.
Material and methods
Control group
A control group comprised 78 patients treated with classical surgery method at the Clinic of Vascular Surgery and Angiology in 1995-2002 due to the abdominal aortic aneurysms and aortoiliac occlusive disease. The patients were qualified for surgery based on clinical and ultrasound evaluation. The group included 50 patients with simple aneurysms located below the renal arteries, 5 patients with ruptured aneurysms and 23 with impaired patency of the aorta and the iliac arteries, who belonged to the same risk group in terms of evaluation of the visceral flow hemodynamics.
Studied group
Qualification started in reference to 60 patients treated with aortoiliac stent graft implantation due to abdominal aortic and iliac artery aneurysm. Initially, 10 patients were evaluated (fig. 2).
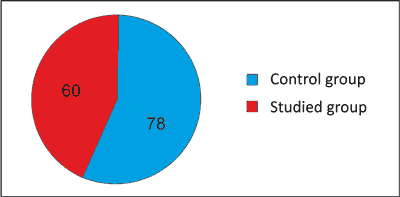
Fig. 2. Material.
Methods
Previous experience gained in evaluation of the control group was transferred to a current studied group in order to conduct comparative analysis.
All fasted patients underwent standard CDD ultrasound study, which was focused on results of morphological and functional evaluation of the visceral arteries and the iliac arteries. Ultrasound machine Siemens Antares Premium and a broad-band probe convex 5 MHz were used.
Basic study includes evaluation of the coeliac trunk (CT), the superior mesenteric artery (SMA), the inferior mesenteric artery (IMA), and the internal iliac arteries (IIA).
Morphological evaluation includes detecting and establishing percent of stenosis of the visceral artery.
Functional evaluation includes quantitative and qualitative assessment of flow with support of analysis of the blood flow velocity using Fourier analysis curve, automatically performed by the ultrasound machine.
Quantitative assessment relates to the level of the systolic velocity (SV), average velocity (AV), end diastolic velocity (EDV), resistance index (RI), and pulsation index (PI).
Qualitative assessment (nature of the flow) includes detecting flow direction, grouping velocity components above or below the baseline, recognizing high resistance flow or low resistance flow, and establishing type of a flow phase (single-phase, two-phase, three-phase).
Using so-called ultrasound ischemia ratios established in a control group (velocity ratio of ischemia (VRI) and resistance ratio of ischemia (RRI)), it was planned that after evaluation of the whole material, the risk of a potential ischemia would be determined and form of ischemia would be predicted (occlusive or non-occlusive ischemia).
Results
10 patients, who underwent endovascular aneurysm repair (EVAR) procedure, have been examined so far. In 70% of patients stent graft was implanted into the aorta and the common iliac arteries, and in 30% of patients it was implanted into the aorta and the external iliac arteries (EIA) (fig. 3).
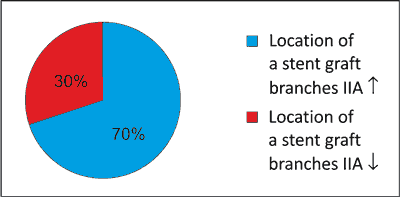
Fig. 3. Classification of the studied group by type of the stent graft.
IMA was initially patent in 80% of patients. Similar results were obtained in the control group. In classical surgery, the straight prosthesis was implanted in 75%, and branched prosthesis in 25% of the patients (fig. 4), and IMA was patent in 93% of cases.
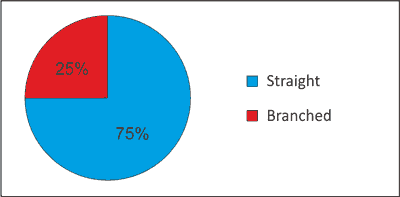
Fig. 4. Classification of the control group by type of the prosthesis.
Based on results of ultrasound evaluation, it was predicted that symptoms of ischemia in the pelvis and the colon would occur in all patients with the prosthesis, which branches were placed in EIA (30%) – IIA coverage. The risk of ischemia was established by visualizing common iliac artery aneurysms reaching the orifices of IIA.
The risk group included the patients with SMA stenosis exceeding 60%. High degree stenoses in SMA were not detected. Small and medium stenoses located in the initial segments, were caused by laminae coming out of the aortic wall.
Superiority in flow parameters of IMA over SMA was established before surgery in 3 patients. No significant morphological and functional changes were detected in CT; therefore, the role of this artery was omitted in perfusion of the pelvis and the colon.
Based on clinical evaluation, signs of ischemia in pelvis were established in 2 patients in form of pelvic claudication. Claudication and postoperative diarrhoea without signs of rectal bleeding occurred in 1 patient. The diarrhoea receded after 5 days. In all 3 patients with mild symptoms of ischemia (30%), the claudication was present on the day of discharge from the hospital. The symptoms occurred in 2 patients with the prosthesis in EIA and in 1 patient due to primary occlusion and stenosis in IIA.
Stenoses in IIA were located in places of branching off. Single aneurysm of the right IIA was located, and then it was covered with a branch. In the control group, half of the patients revealed abnormalities within IIA: stenoses, one- or two-sided occlusions, aneurysms.
In the studies group, we established that there was no difference in flow parameters in the arteries without stenoses and in the ones with stenoses comparing to the control group, and similarly, statistically significant increase in systolic velocity in SMA and its statistically significant increase or decrease in IMA and decrease in IIA, comparing to reference value for healthy subjects.
Similarly, RI values in CT, SMA, and IIA in both groups oscillated at higher levels comparing to reference value for healthy subjects. In cases, where parametric superiority of IMA occurs, the flow reveals low-resistance, and in SMA it reveals high-resistance. No other differences in reference to IMA were observed comparing to reference value for healthy subjects.
Before a surgery, it was observed in patients at risk of ischemia, who belonged to the control group, that flow in IMA revealed signs of low-resistance, and in SMA, it revealed signs of high-resistance. These patients demonstrated the highest number of indications for repeated implantation into IMA.
In 3 patients from the studied group, symptoms of ischemia were confirmed by results of ultrasound measurements of ratios of ischemia (VRI and RRI).
Detected values of PI in both groups oscillated at the similarly higher levels than it did in healthy subjects.
Discussion
In studied group, ultrasound evaluation is a method of choice in detection and control of AAA (14). Specificity and sensitivity of ultrasound evaluation in detection of AAA reaches 100%. Disadvantage of ultrasound method is the fact that the aorta and its collaterals are not visible due to obesity or flatulence. There is a phenomenon of variable evaluation of the diameter and morphology of the wall depending on experience of an evaluating person. Morphological evaluation of suprarenal and subrenal part, para-aortic area and detection of iliac artery aneurysms are applicable (9, 15-17). If there is imminent need to perform surgical intervention, we perform basic preoperative evaluation – CT angiogram. CT angiogram allows selecting surgical method (classical, endovascular) and correct measurement in order to select an appropriate prosthesis for EVAR procedure (18).
In 30-day observation following classical surgery, general mortality rate is approximately 1-8%. Rate of other complications reaches 13-40%. Disturbance of intestinal function, ischemia and occlusion occur in 2-11% of cases (19). Special complication with general mortality rate of 2% is a colonic ischemia. Incidence of postoperative colonic ischemia reaches 11-36% after classical elective surgeries and 40-60% after surgeries performed in ruptured aneurysms (20). Mortality rate is 50% after elective surgeries and 90% after surgeries performed in ruptured aneurysms (11, 21, 22).
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Mayer D, Rancic Z, Pfammatter T et al.: Choice of treatment forthepatient with urgent AAA: practical tips. J Cardiovasc Surg 2009; 50 (5): 595-598.
2. Franks S, Lloyd G, Fishwick Get al.: Endovascular treatment of ruptured and symptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2006; 31: 345-350.
3. Hobo R, Sybrandy JE, Harris PL, Buth J: EUROSTAR Collaborators: Endovascular reapir of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR Experience. J Endovasc Ther 2008; 15 (1): 12-22.
4. Powell JT: Ten years after the EVAR I trial: what have the patients been through? [In:] Becquemin J-P: (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 208-211.
5. Giles KA, Schermerhorn ML, O’Malley AJ et al.: Risc prediction for perioperative mortality of endovascular vs open repair of abdominal aortic aneurysms using the Medicare population. J Vasc Surg 2009; 50: 256-262.
6. Prinssen M, Verhoeven EL, Buth J et al.: Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004; 351: 1607-1618.
7. Visser JJ, van Sambeek MR, Hamza TH et al.: Ruptured abdominal aortic aneurysms endovascular repair versus open suregery – systematic review. Radiology 2007; 243: 122-129.
8. Becquemin J-P, Majewski M, Fermani N et al.: Colon ischemia following abdominal aneurysms repair in the era of endovascular aortic repair. J Vasc Surg 2008; 47: 258-263.
9. Schlösser FJV, Moll FL: Epidemiology. [In:] Management of abdominal AAA. Clinical Practise Guidelines of the ESVS. Eur J Vasc Ebdovasc Surg 2011; 41 (Suppl 1): 16.
10. Verhoeven EL, Oikonomou K: Duplex scan is sufficient. [In:] Becquemin J-P: (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 279-284.
11. Dąbek P: Nieinwazyjna dopplerowska ocena ukrwienia okrężnicy esowatej u chorych z tętniakiem brzusznego odcinka aorty i niedrożnością tętnic biodrowych – Rozprawa doktorska. Warszawa 2002; 32-33.
12. Björck M, Bergqvist D: Blood-flow of the inferior mesenteric and internal iliac arteries among patients undergoing open surgery for abdominal aortic aneurysm. VASA 2006; 35: 11-14.
13. Dąbek P, Hendiger W, Słowiński P et al.: Czy ultrasonograficzna ocena wyników stentowania tętnic kończyn dolnych jest uzasadniona? Ultrasonografia 2012; vol 11, 48.
14. Lindholt JS, Vammen S, Juul S et al.: The validity of ultrasonographic scanning as a screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999; 17: 472-475.
15. Iezzi R, Basilico R, Giancristofaro D et al.: Contrast-enhanced ultrasound versus color duplex ultrasound imaging in the follow-up of patients after endovascular abdominal aortic aneurysms repair. J Vasc Surg 2009; 49 (3): 552-560.
16. Sun Z: Diagnostic value of color duplex ultrasonografy in the follow-up of endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol 2006; 17: 759-764.
17. Chaer RA, Gushchin A, Rhee R et al.: Duplex ultrasound as the sole long-term surveillance method post-endovascular aneurysms repair: a safe alternative for stable aneurysms. J Vasc Surg 2009; 49 (4): 845-849.
18. Jean-Baptiste E, Hassen-Khodja R: CT scan is still mandatory. [In:] Becquemin J-P, editor. Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 272-278.
19. Schlösser FJV, Moll FL: Epidemiology. [In:] Management of abdominal AAA. Clinical Practise Guidelines of the ESVS. Eur J Vasc Ebdovasc Surg 2011; 41 (Suppl 1): 18-20.
20. Björck M, Bergqvist D, Troëng T: Incidence and clinical presentation of bowel ischaemia after aortoiliac surgery-2930 operations from a population-based registry in Sweden. Eur J Vasc Endovasc Surg 1996; 12: 139-144.
21. Sandison AJ, Edmondson RA, Panayiotopoulos YP et al.: Fatal colonic ischemia after stent graft for aortic. Eur J Vasc Endovasc Surg 1997; 13 (2): 472-475.
22. Björck M, Troëng T, Bergqvist D: Risc-factors for intestial ischaemia after aortoiliac surgery. A combined cohort and case control study of 2824 operations. Eur J Vasc Endovasc Surg 1997; 13: 531-539.
23. Schlösser FJV, Moll FL: Epidemiology. [In:] Management of abdominal AAA. Clinical Practise Guidelines of the ESVS. Eur J Vasc Ebdovasc Surg 2011; 41 (Suppl 1): 20-22.
24. Buth J, Laheij RJ: Early complications and endoleaks after endovascular abdominal aortic aneurysms repair: report of a multicenter study. J Vasc Surg 2000; 31 (1 Pt 1): 134-146.
25. Wassélius J: Is PET-scan useful to predict AAA rupture? In: Becquemin J-P, editor. Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 212-215.
26. Makar RR, Badger SA, O’Donnell ME et al.: The effects of abdominal compartment hypertension after open and endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2009; 49: 866-872.
27. Djavani K, Wanhainen A, Björck M: Intra-abdominal hypertension and abdominal compartment syndrome following surgery for ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2006; 31: 581-584.
28. Rodriguez-Lorenzo L, Vila-Coll R, Iborra-Ortega E et al.: EVAR for ruptured AAA: results in real life. [In:] Becquemin J-P, editor. Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 225-232.
29. Björck M: Can we reduce the rate and severity of colonic ischemia followin AA-repair? [In:] Becquemin J-P: (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 233-239.
30. Champagne BJ, Lee EC, Valerian B et al.: Incidence of colonic ischemia after repair of ruptured abdominal aortic aneurysm with endograft. J Am Coll Surg 2007; 204 (4): 597-602.
31. Senapati A, Browse NL: Gluteal necrosis and paraplegia following postoperative bilateral internal iliac artery occlusion. J Cardiovasc Surg 1990; 31: 194-196.
32. Dadian N, Veith FJ, Ohki T et al.: Overt colon ischemia after endovascular aneurysms repair: the importance of microembolization as an etiology. J Vasc Surg 2001; 34: 986-996.
33. Malinzak LE, Long GW, Bove PG et al.: Gastrointestinal complications following infrarenal endovascular aneurysm repair. Vasc Endovascular Surg 2004; 38 (2): 137-142 (Abstract).
34. Budzyński J, Wasilewski M, Wiśniewska J et al.: Ostre niedokrwienie jelit – czy nadszedł czas na 24-godzinne dyżury w pracowni angiograficznej? Rola leczenia wewnątrznaczyniowego. Acta Angiologica 2011; 17 (4): 237-250.
35. Björck M, Wanhainen A: Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg 2010; 23 (1): 54-64.
36. Welborn MB 3rd, Seeger JM: Prevention and management of sigmoid and pelvic ischemia associated with aortic surgery. Semin Vasc Surg 2001; 14 (4): 255-265.
37. Loftus I: Branched grafts for the hypogastric artery: when is it feasible and is it durable? [In:] Becquemin J-P: (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 267-271.
38. Unno N, Inuzuka K, Yamamoto N et al.: Preservation of pelvic circulation with hypogastric artery bypass in endovascular repair of abdominal aortic aneurysm with bilateral iliac artery aneurysms. J Vasc Surg 2006; 44: 1170-1175.
39. Schneider PA: Tips for managing bad iliac arteries during EVAR. [In:] Becquemin J-P, (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 243-247.
40. Cochennec F, Desgranges P, Allaire E et al.: They must be preserved. [In:] Becquemin J-P, (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 262-266.
41. Mehta M, Veith FJ, Cayne NS et al.: Bilateral hypogastric interruption is safe when needed during EVAR: Outcomes of a multicenter 135 patient study. J Vasc Surg 2009; 50(4): 964.
42. Veith FJ, Mehta M, Cayne NS et al.: They can be covered or occluded safely – even bilaterally. [In:] Becquemin J-P, (ed.) Controversies and updates in vascular surgery. Torino: Edizioni Minerva Medica 2011; 256-261.




