© Borgis - Postępy Nauk Medycznych 11/2012, s. 837-842
*Jarosław Kozakowski, Michał Rabijewski, Wojciech Zgliczyński
Zależność między masą tłuszczu brzusznego i gynoidalnego a wskaźnikami metabolicznymi i stężeniem androgenów u otyłych kobiet z PCOS
Association between abdominal and gynoid fat mass, metabolism markers and serum androgens in obese women with polycystic ovary syndrome**
Department of Endocrinology, Medical Center of Postgraduate Education, Warsaw
Head of Department: prof. Wojciech Zgliczyński, MD, PhD
Streszczenie
Cel pracy. Ocena zależności między masą tłuszczu brzusznego i gynoidalnego a wskaźnikami metabolizmu węglowodanów i lipidów oraz androgenami w surowicy u otyłych kobiet z zespołem policystycznych jajników (PCOS).
Materiał i metody. U 20 kobiet z PCOS w wieku 19-49 lat, z BMI 27,3-53,8 kg/m2 dokonano pomiarów antropometrycznych oraz określono na czczo stężenie glukozy, lipidów, insuliny, leptyny, LH, FSH, estradiolu, androgenów, SHBG, fT4 i TSH. Skład ciała oceniono metodą DEXA.
Wyniki. U wszystkich badanych stwierdzono zwiększoną masę tłuszczu brzusznego oraz hiperandrogenizm, u siedmiu hiperinsulinemię, a u piętnastu oporność insulinową. Wykazano dodatnią korelację między BMI, masą tłuszczu brzusznego i obwodem talii (WC) a stężeniem triglicerydów (odpowiednio: r = 0,45, p < 0,05; r = 0,45, p < 0,05; r = 0,56, p < 0,01), insuliny (odpowiednio: r = 0,79, p < 0,001; r = 0,61, p < 0,01; r = 0,71, p < 0,01) i skurczowym ciśnieniem tętniczym (odpowiednio: r = 0,68, p < 0,001; r = 0,59, p < 0,01; r = 0,58, p < 0,01). Stężenie leptyny korelowało z ciężarem ciała (r = 0,68, p < 0,05), BMI (r = 0,67, p < 0,05), masą tłuszczu całkowitą (r = 0,62, p < 0,05), i WC (r = 0,83, p < 0,01). Nie stwierdzono bezpośredniej korelacji między wskaźnikami otyłości a stężeniem hormonów płciowych. Wykazano korelację między stężeniem androgenów: androstendionu i DHEA-S a TSH (odpowiednio: r = 0,61, p = 0,0065 i r = 0,64, p = 0,01).
Wnioski. DEXA jest wartościową metodą oceny składu ciała u kobiet z PCOS. Z jej zastosowaniem u badanych stwierdzono zwiększoną masę tłuszczu brzusznego. Wykazano dodatnią korelację między masą tłuszczu w jamie brzusznej a stężeniem trglicerydów i insuliny w surowicy oraz ciśnieniem tętniczym krwi. Nie stwierdzono bezpośredniej korelacji między wskaźnikami otyłości a stężeniem hormonów płciowych, natomiast wykazano zależność między stężeniem hormonów androgenowych i TSH.
Summary
Aim. To evaluate associations between abdominal and gynoid fat, glucose and lipid metabolism markers and serum androgens in obese women with polycystic ovary syndrome (PCOS).
Material and methods. In 20 women with PCOS aged 19-49 years with body mass index (BMI) 27.3-53.8 kg/m2 anthropometric measurements were performed. Fasting serum glucose, lipids, insulin, leptin, LH, FSH, estradiol, androgens, SHBG, fT4 and TSH were estimated. Body composition was measured by DEXA scan.
Results. All of the subjects had increased abdominal fat and were hyperandrogenic, seven of them had elevated fasting serum insulin levels, and fifteen were insulin resistant. BMI, abdominal fat and waist circumference (WC) positively correlated with triglycerides (r = 0.45, p < 0.05; r = 0.45, p < 0.05; r = 0.56, p < 0.01, respectively), insulin (r = 0.79, p < 0.001; r = 0.61, p < 0.01; r = 0.71, p < 0.01, respectively), and systolic blood pressure (r = 0.68, p < 0.001; r = 0.59, p < 0.01; r = 0.58, p < 0.01, respectively). We found a correlation between leptin levels and body weight (r = 0.68, p < 0.05), BMI (r = 0.67, p < 0.05), total fat (r = 0.62, p < 0.05) and WC (r = 0.83, p < 0.01). No direct correlation between fat mass indices and sex hormones were found. We observed a correlation between androgens and TSH: androstendione (r = 0.61, p = 0.0065) and DHEA-S (r = 0.64, p = 0.01).
Conclusions. DEXA is a valuable method of body composition assessment in women with PCOS. Studied subjects had abdominal type of obesity. There was a positive correlation between abdominal obesity and cardiovascular risk factors: triglyceride and insulin levels and blood pressure. We did not prove any direct association between fatness and serum androgens but correlation between androgens and TSH was found.

Introduction
Polycystic ovary syndrome (PCOS) is the most frequent endocrine disorder in women in the reproductive age and is present in 5-10% of them (1). Criteria for diagnose this syndrome were worked out by the international consensus conference in Rotterdam in 2003 (2). Women with PCOS present clinical heterogeneity, although insulin resistance, disturbed ovarian and adrenal steroidogenesis with menstrual irregularity and polycystic ovarian morphology are most frequent features (3-5).
Obesity, that influences the phenotypic expression of PCOS in approximately 50% of patients (6) is known to be metabolically active, and leads to insulin resistance, subsequent hyperinsulinemia, increased lipolysis and release of free fatty acids from fat cells. However, insulin resistance was found also in lean women with PCOS (7). Abdominal fat secrets several metabolic factors, with proinflammatory cytokines among them. Also androgen excess and fertility disorders may be associated with obesity in these women (8).
A number of different methods to investigate body composition have been worked out. Anthropometric noninvasive measures are widely use because of their simplicity and convenience. Techniques of direct measure of adiposity (e.g. computed tomography, total body water, total body potassium) have important limitations: exposure to ionizing radiations, high cost and methodological complexity. In our study we used dual-energy x-ray absorptiometry (DEXA), that allows to measure accurately both total and regional fat with marginal exposure to radiation.
Aim of study
The aim of our study was to evaluate associations between abdominal and gynoid fat, glucose and lipid metabolism markers, blood pressure and serum androgen levels in overweight and obese women with polycystic ovary syndrome.
Material and methods
20 women with PCOS, aged 19-49 years, mean 30.3 ± 8.6 (x ± SD) with BMI 27.3-53.8 kg/m2, mean 38.02 ± 6.5 were included into the study. All of the women were obese except one that was overweight. The diagnosis of PCOS was based on criteria of the Rotterdam consensus: at least two of the following features: 1) oligomenorrhea or amenorrhea, 2) clinical and/or biochemical evidence of hyperandrogenemia and 3) polycystic ovaries in ultrasound imaging.
Biochemical hyperandrogenemia was defined as serum testosterone levels greater than 0.9 ng/ml, androstendione levels greater than 310 ng/dl and dehydroepiandrosterone-sulfate levels greater than 2000-4100 ng/ml, depending on age. Ovary in USG were defined as polycystic when they included either 10 or more follicles measuring 2-9 mm in diameter or their volume was greater than 10 cm3.
The exclusion criteria included hypothyroidism, hyperprolactinemia, Cushing’s syndrome, nonclassical congenital adrenal hyperplasia and current or previous (within the last 3 months) use of oral contraceptives and other hormonal, antidiabetic and antiobesity drugs.
A screening consisted of full physical examination, laboratory tests and imaging. Patients were examined after an overnight fast. Waist circumference, body height and weight were assessed, and then body mass index (BMI) was calculated. Blood was collected at about 8.00 h for glucose, lipids (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglicerydes), insulin, leptin, LH, FSH, estradiol, testosterone, androstendione, dehydroepiandrosterone-sulfate, sex hormone-binging globulin (SHBG), free tyroxine and TSH through an iv catheter placed in the forearm. HOMA index was calculated by the formula: fasting plasma insulin (microinternational units per milliliter) x fasting plasma glucose (millimoles per liter)/22.4. Usually the other day all of the subjects underwent transvaginal ultrasonography (TV-USG) and USG of abdomen (to exclude adrenal pathology). Body composition was determined by DEXA. The same two operators performed all TV-USG and DEXA measurements, respectively.
Assays
Insulin was measured by immunoradiometric method (Insulin IRMA – Immunotech SA, France); sensitivity was 2.0 mIU/ml. Leptin was measured by RIA (Linco Res. Inc, USA), using rabbits antibodies against human leptin. The sensitivity for this assay was 0.5 ng/ml. LH, FSH and TSH were measured by immunochemiluminescence method with IMMULITE 2000 (Siemens Healthcare Diagnostics, Inc). Estradiol was measured with the same IMMULITE 2000 analyzer; sensitivity was 15 pg/ml. Total testosterone was measured by RIA-CT method (Immunotech SA, France); sensitivity of this method was 0.025 ng/ml. Androstendione was measured by direct RIA-CT (DSL, USA). Dehydroepiandrosterone-sulfate was measured by RIA-CT method (Spectria, Orion Diagnostica, Finland); sensitivity of this method was 10 ng/ml. Prolactin was measured by IMMULITE 2000 (Siemens Healthcare Diagnostics, Inc) sensitivity of the method was 0.01 μg/mL.
Body mass index was calculated as a body weight (kg)/height (m2). To perform measurements of body composition by DEXA we used a region of interest (ROI) program. In this method abdominal fat is estimated in region between the upper part of the pelvis with the upper margin 96 mm superior to the lower part of this region. The lateral part of this region is defined by the lateral part of the thorax. The upper part of the gynoid fat region is defined by the superior part of trochanter major, with the lower margin 96 mm inferior to the upper part of the trochanter major. The lateral part of this region is defined by the subcutaneous tissue on the hip, which can be visualized using the Image Values option (fig. 1). We used Lunar Prodigy (GE Lunar, Madison, WI, USA) equipment, which was calibrated each day with a standardized phantom and serviced regularly. The coefficient of variation for measurements of body composition with this method is about 2%.
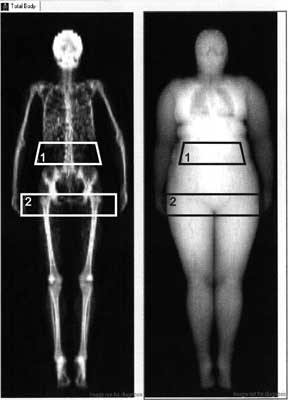
Fig. 1. Example of the regions of interest (ROI) delimiting abdominal (1) and gynoid (2) fat in one of our studied obese woman with polycystic ovary syndrome.
Statistical analysis
All the data are presented as the mean ± SD. The normality of the distribution of variables was verified with a Kolmogorov-Smirnov and Lilieforse tests. To examine bivariate relationships between data Pearson correlation or Spearman rank analyses were used. Comparisons between groups with normal distribution of the data were performed by unpaired Student’s t-test, in other cases comparisons were performed by Kolmogorov-Smirnov test for two samples. For all analysis, a two-tailed P ≤ 0.05 was considered to indicate statistic significance.
Results
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Carmina E, Lobo RA: Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 1999; 84: 1897-1899.
2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risk related to polycystic ovary syndrome. Fertil Steril 81: 19-25.
3. Dunaif A: Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997; 18: 774-800.
4. DeUgarte CM, Bartolucci AA, Azziz R: Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005; 83: 1454-1460.
5. Hoffman LK, Ehrmann DA: Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 2008; 4: 215-222.
6. Gambineri A, Pelusi C, Vicennati V et al.: Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002; 26: 883-896.
7. Guzick DS: Cardiovascular risk in PCOS. J Clin Endocrinol Metab 2004; 89: 3694.
8. Pasquali R: Obesity and androgens: facts and perspectives. Fertil Steril 2006; 85: 1319-1340.
9. Yildrim B, Sabir N, Kaleli B: Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1358-1364.
10. Puder JJ, Varga S, Kraenzlin M et al.: Central fat excess in polycystic ovary syndrome: relation to low grade inflammation and insulin resistance. J Clin Endocrinol Metab 2005; 90: 6014-6021.
11. Faloia E, Canibus P, Gatti C et al.: Body composition, fat distribution and metabolic characteristics in lean and obese women with polycystic ovary syndrome. J Endocrinol Invest 2004; 27: 424-429.
12. Zimmermann S, Phillips RA, Dunaif A et al.: Polycystic ovary syndrome: lack of hypertension despite profound insulin resistance. J Clin Endocrinol Metab 1992; 75: 508-513.
13. Holte J, Gennarelli G, Berne C et al.: Elevated ambulatory day-time blood pressure in women with polycystic ovary syndrome: a sign of a pre-hypertensive state? Hum Reprod 1996; 11: 23-28.
14. Carmina E, Bucchieri S, Esposito A et al.: Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extend of its relation to insulin resistance. J Clin Endocrinol Metab 2007; 92: 2500-2505.
15. Holte J, Bergh T, Gennarelli G, Wide L: The independent effects of polycystic ovary syndrome and obesity on serum concentrations of gonadotrophins and sex steroids in premenopausal women. Clin Endocrinol (Oxf) 1994; 41: 473-481.
16. Holte J, Bergh T, Berne C, Lithell H: Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf) 1994; 41: 463-471.
17. Silfen ME, Denburg MR, Manibo AM et al.: Early endocrine, metabolic and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescents. J Clin Endocrinol Metab 2003; 88: 4682-4688.
18. Poppe K, Velkeniers B, Glinoer D: Thyroid disease and female reproduction. Clin Endocrinol (Oxf) 2007; 66(3): 309-321.
19. Janssen OE, Mehlmauer N, Hahn S et al.: High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol 2004; 150: 363-369.
20. Wajchenberg PL: Subcutaneous and visceral adipose tissue: their relation to metabolic syndrome. Endocr Rev 2000; 21: 697-738.

