© Borgis - Postępy Nauk Medycznych 3/2015, s. 166-172
*Beata Bergler-Czop1, Ligia Brzezińska-Wcisło1, Bartosz Miziołek2
Pemfigoid pęcherzowy w oddziałach dermatologii województwa śląskiego w latach 2001-2014
Bullous pemphigoid in Dermatology Departments of Silesia in years 2001-2014
1School of Medicine in Katowice, Medical University of Silesia in Katowice, Department of Dermatology
Head of Department: prof. Ligia Brzezińska-Wcisło, MD, PhD
2Andrzej Mielęcki Silesian Independent Public Clinical Hospital in Katowice, Department of Dermatology
Head of Department: prof. Ligia Brzezińska-Wcisło, MD, PhD
Streszczenie
Wstęp. Pemfigoid pęcherzowy (pemphigoid bullosus) jest najczęściej występującym schorzeniem z grupy chorób pęcherzowych. Dotyczy zwykle osób starszych, ryzyko względne zachorowania w 90. r.ż. jest blisko 300-krotnie wyższe niż w 60. r.ż. W 15-20% przypadków pemfigoid pęcherzowy może być rewelatorem paraneoplastycznym – opisywano przypadki typowych pęcherzowych zmian skórnych zarówno poprzedzających rozwój, jak i współistniejących z nowotworami.
Cel pracy. Celem pracy była ocena częstości występowania pemfigoidu w różnych grupach wiekowych, metod i wyników leczenia, współistnienia z chorobami nowotworowymi i innymi schorzeniami dodatkowymi, wpływu leków stosowanych przez pacjentów na etiologię pemfigoidu.
Materiał i metody. Analizą objęto 120 pacjentów z rozpoznanym pemfigoidem pęcherzowym: 74 kobiety oraz 46 mężczyzn. Średnia wieku pacjenta wynosiła 71,13 ± 11,8 roku.
Wyniki. Współistnienie innych schorzeń układowych stwierdzono u 87,11% pacjentów, z czego u 19,8% wywiad w kierunku schorzeń nowotworowych był dodatni. Lekiem z wyboru w terapii pemfigoidu pozostawał Metyloprednizolon. Wśród innych opcji terapeutycznych stosowano również glikokortykosteroidy (Deksametazon, Prednizon, Triamcynolon), leki immunosupresyjne (Cyklofosfamid, Chlorambucil, Azatiopryna), antybiotyki (Erytromycyna, Doksycyklina, Ceftriakson) oraz witaminę PP i Dapson.
Wnioski. Obraz kliniczny pemfigoidu pozostaje zróżnicowany, a rozpoznanie choroby powinno być zawsze uzupełnione o diagnostykę w kierunku ewentualnych, ukrytych schorzeń nowotworowych. Chociaż glikokortykosteroidy pozostają terapią z wyboru, to konieczne są dalsze badania, które pozwolą na lepsze poznanie i ugruntowanie skuteczności innych opcji terapeutycznych.
Summary
Introduction. Bullous pemphigoid (BP) is the most common disease from a group of bullous dermatoses. It affects mostly elderly people and the relative risk of disease development in people at the age 90 is 300-fold higher than in individuals who are 60 years old. It is supposed, that 15-20% of patients with BP may have malignant internal tumor and skin lesions can even precede the recognition of malignancy.
Aim. The aim of this retrospective study was to assess the occurrence of BP in relation to age of patients and concomitant systemic diseases, including neoplastic disorders. Further analysis gives a review of therapeutic options and the efficacy of these modalities.
Material and methods. A study group comprised of 120 patients who were diagnosed with BP: 74 female and 46 male patients. The mean age of patient was 71.13 ± 11.8.
Results. Co-existing of other disease was reported in 87.11% of patients and the history towards neoplastic disorder (active or previously treated) was positive in 19.8% of patients. In therapy of the disease, the drug of choice was Methylprednisolone. Other therapeutic options were also glucocorticoids (Dexamethasone, Prednisone, Triamcinolone), immunosuppressive agents (Cyclophosphamide, Chlorambucil or Azathioprine), antibiotics (Erythromycin, Doxycycline, Ceftriaxone), niacin and Dapsone.
Conclusions. Clinical presentation of BP is distinguished and the recognition of the disease should be always supplemented by diverse diagnostic tests towards potent and hidden tumor. Although, glucocorticoids have remained a first choice therapy with good effects, further studies are necessary to establish the efficacy of other therapeutic modalities.

Introduction
Bullous pemphigoid (BP) is the most common disease from a group of bullous dermatoses and it affects mostly elderly people who are over 70 years old (1). An etiology of the disease has been still not fully elucidated, however some autoimmune antibodies against components of the basement membrane zone (BMZ) are suggested to be the main factors.
An onset of the disease can be non-specific and some urticarial-type or eryhematous lesions can occur on the skin. Following, bullae formation arise and may develop on previously unchanged skin or in area of preexisting, non-specific skin eruption. Typically, bullae are tense, firm-topped with epidermal layer and may contain serous or hemorrhagic fluid. The originally tense bullae rupture and transform into erosion which is covered with hemorrhage crusts. Some lesions can be accompanied by itching. The disease mostly spares mucosae membranes, however in 20-30% of patients some lesions occur also in oral cavity (fig. 1-5).
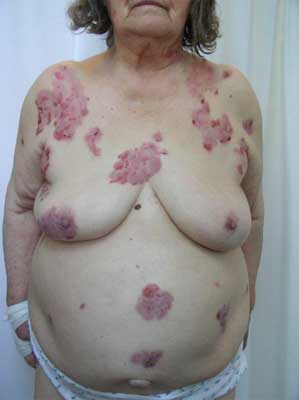
Fig. 1. 85-year-old patient, woman, eruptions numerous, well-tensed bullas on the erythematous area, edemic erythemas as well as erosions covered with hemorrhage crusts on the patient’s torso, lower and upper extremities.
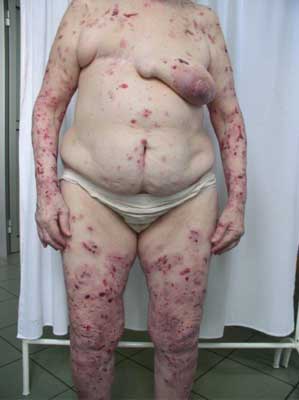
Fig. 2. 65-year-old patient, woman, erythema, bullas and blisters with a well-tensed surface, erosions, located near the scar after right mastectomy.
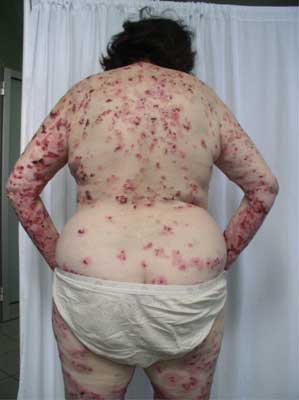
Fig. 3. 65-year-old patient, woman, erythema, bullas and blisters with a well-tensed surface, erosions, located on the back.
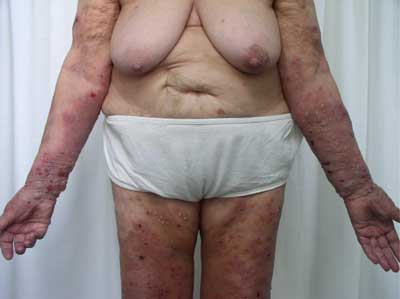
Fig. 4. 81-year-old patient, woman, numerous, well-tensed blisters on erythematous base, edemous erythemas and erosions covered with hemorrhagic eschars found on the patient’s torso, upper and lower limbs.
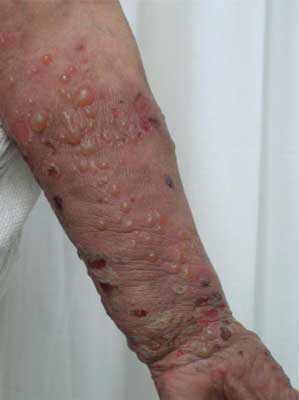
Fig. 5. 81-year-old patient, woman, well-tensed blisters on erythematous base, edemous erythemas and erosions covered with hemorrhagic eschars found on the patient’s upper limb.
Aim
The aim of the analysis was to assess the occurrence of pemphigoid and its correlations to age of patients and association with systemic diseases, including neoplastic disorders.
Material and methods
This is a retrospective study that encompasses the analysis of patients diagnosed with pemphigoid that were treated in years 2001-2014 in the Chair and Department of Dermatology at Medical University of Silesia or in other Dermatology Departments in Silesian Province.
Results
A study group comprised of 120 patients who were diagnosed with BP: 74 female and 46 male patients. The mean age of patient was 71.13 ± 11.8 and in female group it was 72.7 ± 11.3, while in male group 62.43 ± 14.25. The mean time of latency from the occurrence of lesions to diagnosis of the disease was 27 months. The recognition of bullous pemphigoid was confirmed by skin biopsy in all patients. Direct immunofluorescence test in skin biopsy was performed in 74 patients (61.6%) and in 48 of them (65%) was positive. Indirect immunofluorescence test was made in all patients and was positive in 84 patients (70.0%).
In therapy of the disease, the drug of choice was Methylprednisolone, which was administered orally (Metypred) in 39 patients or as intravenous pulses (Solu-Medrol) in the next 6 patients. Other reported glucocorticoids were Prednisone (Encorton) in 21 patients, Triamcinolone (Polcortolon) in 7 patients, Dexamethasone orally (Pabi-Dexamethason) in 11 patients or intravenously (Dexaven) in 17 patients. Further modalities encompassed immunosuppressive drugs such as Cyclophosphamide (Endoxan) in 7 cases, Chlorambucil (Leukeran) in 8 cases or Azathioprine (Imuran) in 4 cases. Some of patients were administered with antibiotics and 27 of them were treated with Erythromycin co-administered with niacin. Doxycycline was reported in 18 cases, Ceftriaxone in 8 and Dapsone (Avlosulfon, Disulone) in 18 cases. A monotherapy was rare and 91% of patients received more than one of systemic therapeutic option. At the same time, all patients were treated externally with topical glucocorticoids or emollients.
Co-existing of other disease was reported in 87.11% of patients and encompassed mainly arterial hypertension, coronary heart disease, epilepsy, diabetes mellitus type 2 and hypothyroidism (with autoimmune thyroid disease reported in 1 case). The history towards neoplastic disorder (active or previously treated) was positive in 19.8% of patients. The breast cancer was the most common neoplastic disease and was reported in 9 patients. Some other malignancies were chronic leukocyte leukemia, lung cancer, colon cancer, gastric cancer, renal cancer or prostate cancer. In the study group, 41% of patients had been reported to receive a long-term systemic therapy of concomitant disorders and antihypertensive drugs (Enalapril, Captopril and Furosemide) were the most common pharmacological interventions in the study group.
Discussion
Sub-epidermal bullae in BP are formed in just below the basal layer of keratinocytes and result from proteolysis and disjunction in the upper part of lamina lucida. In more than 95% of patients IgG antibodies occur and are aimed to components of BMZ. They are specific to BPAG2 (type XVII of collagen), which is a trans-membrane component of hemidesmosome, of 180 kDa weight and to BPAG1, protein of 230 kDa weight connected with internal hemidesmosome lamina. However, BPAG2 – an extracellular antigen is considered to be the starting point of the disease.
An initial diagnosis of BP can be based on four clinical criteria that encompass: age over 70 years, absence of cicatricial atrophic lesions, mucosa unaffected by the disease and lack or minor eruption within skin areas of face and neck. Indeed, a skin biopsy is recommended to diagnose BP. Typically, a sub-epidermal blister is visible in a specimen and the whole epidermis comprises its cover. However, the confirmation of the diagnosis should be based on some immunofluorescence technique. Indirect immunofluorescence (IIF) detects auto-antibodies BP 180 and BP 230 in blood serum with the ELISA or immunoblot tests, while direct immunofluorescence (DIF) reveals deposits of C3 constituent of complement and auto-antibodies IgG along cutaneous-epidermal junction in the skin specimen taken from the border line of lesion (2-4).
Essentially, BP can be a significant paraneoplastic revelator and several cases of a coexistence of BP and malignancy have been reported. It is supposed that 15-20% of patients with BP may have malignant internal tumor. Paraneoplastic pemphigoid most commonly coexists with adenocarcinomas of the gastrointestinal tract, predominantly in stomach and colon. Other malignancies may consider the skin, uterus mucous tunic, breast, lungs, salivary and prostate glands or Castelman disease, however they are less frequently diagnosed. Interestingly, the risk of neoplasia in patients with BP is disputable and some authors contest such correlations (5, 6).
Some cases of sudden onset of BP have been reported to be related to pharmacological interventions and considered mainly with the co-administration of antihypertensive- and diuretic-drugs (especially Furosemide) (3).
A prognosis in BP depends on the age, concomitant disorders as well as on Karnofsky score system, but the extension of skin lesions has the minor significance (7). A low albumin level in blood serum is known to be an additional factor which worsens the prognosis (8). Fairley et al. suggest that a level of eosinophils or immunoglobulin IgE concentration are important factors in the course of the disease (9).
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Kneisel A, Hertl M: Bullous pemphigoid: diagnosis and therapy. Wien Med Wochenschr 2014; 137: 43-49.
2. Schulze F, Kasperkiewicz M, Zillikens D, Schmidt E: Bullous pemphigoid. Hautarzt 2014; 64: 931-943.
3. Zilikens D: Choroby pęcherzowe autoimmunologiczne. [W:] Braun-Falco O, Burgdorf WH, Plewig G et al. (red.): Braun-Falco Dermatologia. Tom II. 2 wyd. Wydawnictwo Czelej, Lublin 2010: 663-690.
4. Olasz EB, Yancey KB: Bullous pemphigoid and related subepidermal autoimmune blistering diseases. Curr Dir Autoimmun 2008; 10: 141-166.
5. Serwin AB, Musialkowska E, Piascik M: Incidence and mortality of bullous pemphigoid in north-east Poland (Podlaskie Province), 1999-2012: a retrospective bicentric cohort study. Int J Dermatol 2014; 53: 432-437.
6. Rzany B, Partscht K, Jung M et al.: Risk factors for lethal outcome in patients with bullous pemphigoid: low serum albumin level, high dosage of glucocorticosteroids and old age. Arch Dermatol 2002; 138: 903-908.
7. Jung M, Kippes W, Messer G et al.: Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol 1999; 41: 266-268.
8. Joly P, Courville P, Lok C et al.: Clinical criteria for the diagnosis of bullous pemphigoid: a reevoluation according to immunoblot analysis of patient sera. Dermatology 2004; 208: 16-20.
9. Fairley JA, Baum CL, Brandt DS, Messingham KA: Pathogenicity of IgE in autoimmunity: successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009; 123: 704-705.
10. Kershenovich R, Hodak E, Mimouni D: Diagnosis and classification of pemphigus and bullous pemphigoid. Autoimmun Rev 2014; 13: 477-481.
11. Błaszczyk M: Leczenie pemfigoidu pęcherzowego. Czy miejscowa terapia mocnymi kortykosteroidami stanowi rozwiązanie problemu? Przegl Dermatol 2005; 4: 271-277.
12. Brick KE, Weaver CH, Lohse C et al.: Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol 2014; 71: 92-99.
13. Monnier-Murina K, Du Thanh A, Merlet-Albran S et al.: Bullous Pemphigoid Occurring during Efalizumab Treatment for Psoriasis: A Paradoxical Auto-Immune Reaction? Dermatology 2009; 6: 357-378.
14. Langan SM, Smeeth L, Hubbard R et al.: Bullous pemphigoid and pemphigus vulgaris-incidence and mortality in the UK: population based cohort study. BMJ 2008; 337: a180.
15. Popadic S, Skiljevic D, Medenica L: Bullous pemphigoid induced by penicillamine in a patient with Wilson disease. Am J Clin Dermatol 2009; 10: 36-38.
16. Patsatsi A, Vyzantiadis TA, Chrysomallis F et al.: Medication history of a series of patients with bullous pemphigoid from northern Greece – observations and discussion. Int J Dermatol 2009; 48: 132-135.
17. Izumi R, Fujimoto M, Yazawa N et al.: Bullous pemphigoid positive for anti-BP180 and anti-laminin 5 antibodies in a patient with graft-vs-host disease. J Am Acad Dermatol 2007; 56: 94-97.
18. Daniel E, Thorne JE: Recent advances in mucous membrane pemphigoid. Curr Opin Ophthalmol 2008; 19: 292-297.
19. Yanagi T, Kato N, Yamane N, Osawa R: Bullous pemphigoid associated with dermatomyositis and colon carcinoma. Clin Exp Dermatol 2007; 32: 291-294.
20. Hall TC: Paraneoplastic syndromes: mechanisms. Semin Oncol 1997; 24: 269-276.
21. Fernandes J, Barad P, Shukla P: Association of bullous pemphigoid with malignancy: a myth or reality? Indian J Dermatol 2014; 59: 390-393.
22. Wong DA, Hunt MJ, Stapleton K: IgA multiple myeloma presenting as an acquired bullous disorder. Austr J Dermatol 1999; 40: 31-34.
23. Taniuchi K, Takata M, Matsui C et al.: Antiepiligrin (laminin 5) cicatricial pemphigoid associated with an underlying gastric carcinoma producing laminin 5. Br J Dermatol 1999; 140: 696-700.
24. Ahmed AR, Avram MM, Duncan LM: Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 23-2003. A 79-year-old woman with gastric lymphoma and erosive mucosal and cutaneous lesions. N Engl J Med 2003; 349: 382-391.
25. Ameen M, Pembroke AC, Black MM, Russell-Jones R: Eosinophilic spongiosis in association with bullous pemphigoid and chronic lymphocytic leukaemia. Br J Dermatol 2000; 143: 421-424.
26. Blum A, Wehner-Caroli J, Scherwitz C, Rassner G: Bullous pemphigoid as a paraneoplastic syndrome. A case report in renal-cell carcinoma. Hautarzt 1997; 48: 834-837.
27. Klein T, Rotterdam S, Noldus J, Hinkel A: Bullous pemphigoid is a rare paraneoplastic syndrome in patients with renal cell carcinoma. Scand J Urol Nephrol 2009; 23: 1-3.
28. Oztürkcan S, Ermertcan AT, Sahin MT et al.: Bullous pemphigoid associated with prostate adenocarcinoma. Indian J Dermatol Venereol Leprol 2004; 70: 39-41.
29. Calderoni A, Altermatt HJ, Pirovino M: Autoimmune processes as paranoplastic manifestation in familial breast carcinoma. Dtsch Med Wochenstrr 1994; 36: 1194-1198.
30. Hauschild A, Swensson O, Christophers E: Paraneoplastic bullous pemphigoid resembling erythema gyratum repens. Br J Dermatol 1999; 140: 550-552.
31. Gilmour E, Bhushan M, Griffiths CE: Figurate erythema with bullous pemphigoid: a true paraneoplastic phenomenon? Clin Exp Dermatol 1999; 24: 446-448.
32. Wong KC, Ho KK: Pemphigus with pemphigoid-like presentation, associated with squamous cell carcinoma of the tongue. Australas J Dermatol 2000; 41; 178-180.
33. Reduta T, Laudańska H, Serwin B, Chodynicka B: Bullous pemphigoid coexisting with internal malignancies – report of three cases and review of literature. Przegl Dermatol 2006; 1: 37-41.
34. Demitsu T, Kakurai M, Yoenda K et al.: Localized pemphigoid (Brunsting-Perry type) with IgG antibody to BP180 NC16a domain resembling lupus erythematosus successfully treated with topical tacrolimus theraphy. J Eur Acad Dermatol Venereol 2008; 18: 38-42.
35. Kjellman P, Eriksson H, Berg P: A retrospective analysis of patients with bullous pemphigoid treated with methotrexate. Arch Dermatol 2008; 144: 612-616.
36. Carrozzo M, Arduino PG, Baldovino S et al.: Minocycline in combination with mycophenolate mofetil in oral mucous membrane pemphigoid. Eur J Dermatol 2008; 18: 198-200.
37. Beissert S, Werfel T, Frieling U et al.: A comparison of oral methyloprednisolone plu azathioprine or mycophenolate mofetil fort he treatment of bullous pemphigoid. Arch Dermatol 2007; 143: 1536-1542.
38. Mignogna MD, Leuci S, Piscopo R, Bonovolontà G: Intravenous immunoglobulins and mucous membrane pemphigoid. Ophthalmology 2008; 115: 752.
39. Schmidt E, Bröcker EB, Goebeler M: Rituximab in treatment-resistant autoimmune blistering skin disorders. Clin Rev Allergy Immunol 2008; 34: 56-64.
40. Stanley JR: Bullous Pemphigoid. [In:] Wolff K, Goldsmith LA, Katz SI et al. (eds.): Fitzpatrick’s Dermatology in General Medicine. 7th ed. McGraw-Hill Companies 2008: 475-480.





