© Borgis - Postępy Nauk Medycznych 5/2015, s. 317-324
Stanisław Winiarczyk1, Jose L. Valverde Piedra2, Sylwia Edyta Szymańczyk3, Katarzyna Szwiec4, 5, Piotr Andrzej Chrościcki6, Jerzy Mackiewicz7, Mateusz Winiarczyk7, Dagmara Wyłupek8, Łukasz Adaszek1, Kamil Torres9, Paulina Świeboda5, Olena Prykhodko4, 5, Olexandr Fedkiv5, Blanka Majda5, Rafał Filip10, Kateryna Goncharova4, 5, 11, *Stefan G. Pierzynowski4, 5, 10
Czy mocznica szczawianowa obserwowana na świńskim modelu ominięcia żołądka Roux-en-Y (RYGB) związana jest z niewydolnością zewnątrzwydzielniczą trzustki?
Is hyperoxaluria in a porcine model of Roux-en-Y gastric bypass (RYGB) associated with exocrine pancreatic insufficiency?
1Department of Epizootiology and Clinic of Infectious Diseases, University of Life Sciences, Lublin, Poland
Head of the Department: prof. Stanisław Winiarczyk, PhD
2Department of Preclinical Veterinary Sciences, University of Life Sciences, Lublin, Poland
Head of the Department: prof. Jose L. Valverde Piedra, PhD
3Department of Animal Physiology, University of Life Sciences, Lublin, Poland
Head of the Department: prof. Iwona Puzio, PhD
4Department of Biology, Lund University, Lund, Sweden
Head of the Department: prof. Christer Löfstedt, PhD
5R&D, SGPlus, Malmö, Sweden
Chief Executive Officer: prof. Stefan G Pierzynowski, PhD
6General Surgery Department of the District Specialist Hospital, Lublin, Poland
Head of the Department: Jerzy Mackiewicz, MD, PhD
7Department of Vitreoretinal Surgery, Medical University of Lublin, Poland
Head of the Department: Andrzej Chrościcki, MD, PhD
8Department and Clinic of Animal Internal Diseases, University of Life Sciences, Lublin, Poland
Head of the Department: Jacek Madany, MD, PhD
9Department of Human Anatomy, Laboratory of Biostructure, Medical University of Lublin, Poland
Head of the Department: prof. Ryszard Maciejewski, MD, PhD
10Institute of Rural Health, Lublin, Poland
Head of the Institute: prof. Iwona Bojar, MD, PhD
11Department of Cytology, Bogomoletz Institute of Physiology, Kiev, Ukraine
Head of Department: prof. Galyna Skibo, MD, PhD
Streszczenie
Wstęp. Niewydolność zewnątrzwydzielnicza trzustki (NZT) z reguły występuje po udanym zabiegu chirurgicznego ominięcia żołądka Roux-en-Y. Jednocześnie jednym z problemów występujących zarówno w przypadku NZT, jak i zabiegów bariatrycznych jest nefropatia szczawianowa.
Cel pracy. Celem badań było tworzenie świńskiego modelu pokarmowej mocznicy szczawianowej po zabiegu ominięcia żołądka, aby określić jego negatywne skutki u ludzi.
Materiał i metody. Badania przeprowadzono na 11 świniach (n = 7 z zabiegiem ominięcia żołądka (Roux-en-Y) i n = 4 nieoperowanych – kontrolne). Świnie były żywione standardową karmą dla świń (SK), karmą zawierającą niską zawartość wapnia lub karmą typu ludzkiego. SK była wzbogacona 0,5 lub 1% szczawianu potasowego (KOx).
Wyniki. Po zabiegu chirurgicznym świnie wykazały całkowite zahamowanie wzrostu przez okres 6 tygodni, po czym zaczęły minimalnie rosnąć. Świnie nieoperowane rosły normalnie w tym czasie. Podczas 51 dni po operacji (okres P1) dzienne wydalanie szczawianów w moczu nie różniło się pomiędzy zwierzętami bariatrycznymi a kontrolnymi i wynosiło 20 ± 10,6 mg/dobę w 51. dniu. Dodanie 0,5% (okres P2) i 1% (okres P3) szczawianu potasu do standardowej paszy dla świń spowodowało wzrost wydalania szczawianów w moczu zarówno u świń bariatrycznych, jak i kontrolnych (odpowiednio: 43,7 ± 32,1 mg/24 dobę vs. 23,2 ± 18,4 mg/24 dobę i 51,4 ± 20,5 mg/24 dobę vs. 48,7 ± 39,2 mg/24 dobę), lecz istotne różnice zaobserwowano tylko po zastosowaniu 1% dodatku do paszy (P < 0,5). Wydalanie szczawianów w moczu utrzymało się na względnie wysokim poziomie u świń RYGB po zaprzestaniu stosowania dodatku szczawianu potasu. Świnie żywione paszą z niską zawartością wapnia wykazywały wyższe poziomy szczawianów w moczu w porównaniu ze zwierzętami żywionymi paszą standardową. Ciekawym odkryciem było stwierdzenie braku jonów wapniowych w moczu świń po zabiegu RYGB.
Wnioski. Szczawiany w diecie odpowiedzialne są za rozwój pokarmowej mocznicy szczawianowej u bariatrycznych świń, które są bardziej wrażliwe niż nieoperowane świnie kontrolne. Opisany w badaniach świński model może być czułym narzędziem do badania mechanizmów pokarmowej mocznicy szczawianowej po zabiegu RYGB u ludzi. Interakcje pomiędzy niewydolnością zewnątrzwydzielniczą trzustki a zabiegami bariatrycznymi powinny być zbadane bardziej szczegółowo.
Summary
Introduction. Exocrine pancreatic insufficiency (EPI) is always provided by efficient Roux-en-Y gastric bypass (RYGB) surgery. In the same time, one of the complications of EPI as well as bariatric surgery is oxalate nephropathy.
Aim. The aim of the study was to develop a porcine model of enteric hyperoxaluria after RYGB to investigate its adverse effects in humans.
Material and methods. A total of 11 pigs (n = 7 Roux-en-Y gastric bypass surgery and n = 4 intact – controls) were used in the study. Pigs were fed either regular pig feed (RF), a low calcium pig feed or human type food. RF was enriched with 0.5 or 1% potassium oxalate (KOx).
Results. Following surgery, pigs displayed complete growth arrest for up to 6 weeks, after which they began to grow slightly. Growth of intact control pigs was normal. During the 51 days following surgery (P1 period), daily urinary oxalate excretion levels did not differ between bariatric and control pigs and was approximately 20 ± 10.6 mg/24 h at day 51. Inclusion of 0.5% (P2 period) and 1% (P3 period) KOx to the regular pig feed, increased urinary oxalate excretion both in bariatric and intact control pigs (43.7 ± 32.1 mg/24 h vs. 23.2 ± 18.4 mg/24 h and 51.4 ± 20.5 mg/24 h vs. 48.7 ± 39.2 mg/24 h, respectively) but significant differences compared to P1 period were found only after the 1% inclusion level (P < 0.05). Oxalate excretion in RYGB pigs remained at relatively higher levels after KOx removal from the diet. Pig fed low calcium diet showed higher levels of urinary oxalate excretion as compared to pigs fed regular diet. Surprisingly total calcium depletion from urine was found in pigs after RYGB.
Conclusions. Dietary oxalate governs the development of enteric hyperoxaluria in bariatric pigs, which are more sensitive to it than intact control pigs. The pig model described in the present study could be a sensitive tool used to highlight the mechanisms of enteric hyperoxaluria following RYGB in humans. The interaction between exocrine pancreatic insufficiency and bariatric surgery should be also more profoundly explored.

Introduction
Roux-en-Y gastric bypass (RYGB) is considered as an effective, acceptable and safe treatment of human obesity (1-3). Sufficient RYGB is mainly dependent on self-digestion on pancreatic enzymes before their contact with digest, which can be compared to the condition of both physiological (neonates, elderly) and pathological exocrine pancreatic insufficiency (EPI). After RYGB patients experience several benefits, including significant weight reduction, reversal of insulin resistance, improvement in hypertension and other cardiovascular risks, and decreased mortality. However, studies have shown that patients undergoing this surgical procedure are at risk for developing hyperoxaluria, nephrolithiasis and oxalate nephropathy (2, 4, 5), among other systemic disorders.
Renal failure due to oxalate nephropathy has been reported by Sinha et al. (4) and Navarro-Díaz et al. (6), who observed that hyperoxaluria may be prevalent in non-stone formers who undergo RYGB surgery as a treatment for weight loss. This has also been observed in other studies, e.g. by Matlaga et al. in which 7.65% of patients were diagnosed with urolithiasis following RYGB surgery, compared to the 4.63% of obese patients in the control group. Moreover, patients after RYGB surgery were more pronounced to undergo shock wave lithotripsy (81 (1.75%) vs. 19 (0.41%)) and ureteroscopy (98 (2.11%) vs. 27 (0.58%)) (7). Furthermore, oxalate nephropathy following RYGB may lead to the development of end stage renal disease (ESRD), thus patients would require dialysis or even renal transplant (8). Importantly, obesity is an independent risk factor for the development of end-stage renal disease (ESRD). Additionally, morbid obesity has been shown to be associated with nephrotic syndrome and it has been reported that proteinuria and segmental glomerulosclerosis can be present in obese patients even in the absence of diabetes (9). On the other hand, both obesity and RYGB surgery are separately associated with an increased risk of kidney deposits (10). Thus, hyperoxaluria together with a high number of renal deposits appears to develop at least 6 months following RYGB surgery (6, 11).
The etiology of post-RYGB hyperoxaluria is not completely understood, but is believed to arise from enteric malabsorption due to reduced small intestine surface area available for nutrient absorption as well as a shortened gastrointestinal transit time. Moreover, limited contact of food with pancreatic enzymes reduces digestion and a malabsorptive state is established, leading to derangements in calcium and oxalate metabolism. Calcium metabolism is altered by its reduced absorption in the bypassed segments of the small intestine. Under normal physiological conditions, free fatty acids in the intestinal lumen saponify calcium, thus preventing the formation of calcium salts (i.e. containing oxalate), instead forming an insoluble product, which is excreted in the faeces (12). However, some fatty acids are believed to promote paracellular intestinal transport and facilitate oxalate transport into the bloodstream (13). What is interesting, EPI condition, which can arise in patients after RYGB surgery (14), can evoke oxalate nephropathy (15). Oxalate nephropathy is an uncommon complication of chronic pancreatitis. The pathophysiological mechanisms, risk factors, and the clinical course of oxalate nephropathy in chronic pancreatitis are poorly known.
To understand the mechanisms of hyperoxaluria development, following bariatric surgery, several animal models have been developed (16, 17). Rats are commonly used for the study of nephrolithiasis (18-21). Despite similarities between human and rodent genomes, there are significant dietary, anatomic, metabolic, and physiological differences (3, 22). In contrast, pigs seem to be a more appropriate model for the study of enteric hyperoxaluria in humans, since pigs and humans are both omnivores and have similarities in the structure and function of the porcine and the human kidneys (23, 24). According to USDA (25) the gastrointestinal tract (GIT) of a 30-40 kg pig has a similar length to that of an adult human and the nutritional requirements of the pig are very similar to humans, however quantitatively there are species differences. Hyperoxaluria in pigs is usually induced by oral administration of hydroxy-L-proline (HLP). The addition of 5% HLP to the diet results in hyperoxaluria, calcium oxalate crystalluria, and nephrolithiasis (26, 27).
The prevalence of hyperoxaluria after RYGB surgery appears to be high, but the incidence of hyperoxaluria-related complications remains unknown. It is of great interest to use an animal model for studying the development of hyperoxaluria to mimic the situation after RYGB in humans.
Aim
The aim of the study was to explore the sensitivity and applicability of the surgical pig model of human Roux--en-Y gastric bypass to study the dependency of enteric hyperoxaluria on dietary oxalate and calcium levels.
Material and methods
Animals
The study made use of 11 growing pigs, 5 to 7 weeks of age and was performed in Poland, Lublin, at the University of Life Sciences and in Sweden, Lund, at Lund University. Pigs were randomly chosen from 2 farms. Six crossbred pigs ((Yorkshire × Swedish Landrace) × Hampshire) were selected from the university herd at Odarslöv, Swedish Agricultural University, and 5 Polish crossbreeds (Polish Landrace x Pietrain) were obtained from a private pig farmer.
Roux-en-Y gastric bypass procedure
At the time of surgery, pigs between 6 and 8 weeks of age. Seven pigs were fasted for 12 hours and premedicated with azaperone (Stresnil; Janssen Pharmaceutica NV, Belgium; 4 mg/kg intramuscularly). Pigs were anaesthetized using a 0.5 to 1.5% air mixture of isoflurane (Forene, isoflurane 100, Abbot Scandinavia AB) and carrier oxygen at approximately 0.5 l/min. An incision was made posterior to the sternum along the linea alba, and the stomach was visualized. From the upper part of the stomach, a small pouch of about 20 mL of volume was created. The lower part of the stomach was closed. For the duodenal loop, the intestine was cut about 30 cm caudally from the pylorus. The jejunum was connected to the stomach pouch, allowing for the passage of feed. About 10 cm caudally from the gastrojejunostomy, the ending of the duodenal loop was connected (end to side). The peritoneum, abdominal muscles, and skin were stitched separately. A silicon catheter was implanted into the right jugular vein (28). The studies were reviewed and approved by the Malmö/Lund Ethics Review Committee on Animal Experiments, Lund’s city court, and the Ethics Committee of the University of Life Sciences in Lublin.
The general study design is shown in figure 1. The pigs were individually housed in metabolic cages, in which they are able to move freely. Each cage had a perforated floor allowing for the collection of urine samples, free of feed and faeces contamination and was equipped with a drinking nipple and a heating lamp. All pigs were acclimated to the metabolic cages for 3 days before the start of the urine collections during particular experimental periods (P1-P6), which lasted: P1: 51, P2: 3, P3: 3, P4: 7, P5: 78 and P6: 47 days, respectively. Body weights were recorded weekly on Mondays before the morning feed.
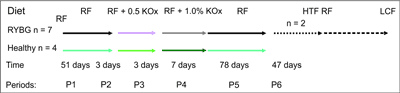
Fig. 1. Study design.
RF – regular feed, KOx – potassium oxalate, HTF – human type of food, LCF – low calcium feed
The mean body weight of pigs at the beginning of the experiment was 12.6 ± 2.5 kg. The experimental group of 7 pigs (4 in Lund and 3 in Lublin) underwent Roux-en-Y gastric bypass surgery performed by the same surgeon. Another group of 4 pigs (2 in Sweden and 2 in Poland) served as intact, healthy controls. Control pigs became part of the experiment 3 weeks after the Roux-en-Y pigs had fully recovered from surgery. Before surgery and until the end of period 4 (P4) – day 64, all 11 pigs were fed regular feed (RF, tab. 1) at a total of 4% of their body weight divided in two meals (for 3 weeks after surgery pigs obtained feed per day in amount of 2% of their body weight). To mimic high amounts of oxalate in the intestines the regular pig feed was enriched with two levels of potassium oxalate (KOx) (0.5 and 1% of the total weight of feed for 3 days; during period P2 and P3, respectively) between days 52 and 57. After KOx diet enrichment, the pigs were fed RF for the next 7 days (period P4). After 80 days of the experiment, all pigs in Lublin (2 control and 3 Roux-en-Y pigs) and 2 controls and 2 Roux-en-Y pigs in Lund were killed and submitted to post mortem autopsy. Two Roux-en-Y pigs in Sweden continued with the experiment for up to 190 days. From day 65 to day 143 (period P5), pigs were fed with human-type food (HTF) that contained vegetables moderately rich in oxalate (peas, cucumber, cabbage) (19, 21) and bread (carbohydrates 50%, protein 10.7%). During period 6 (day 144 to 190), pigs were fed a low calcium dry feed (LCF, tab. 1). Tap water was provided ad libitum during the study, except during urinary collection when 24-hour drinking water intake was recorded.
Table 1. Components of the regular feed (RF) and low calcium feed (LCF).
| Nutrients | Regular feed – RF (%) | Low calcium feed – LCF (%) |
| Protein | 15.5 | 14.1 |
| Fat | 4.3 | 4.7 |
| Fiber | 4.7 | 4.1 |
| Starch | 66.4 | 70.5 |
| Lysine | 1.1 | 1.03 |
| Methionine | 0.38 | 0.49 |
| Ashes | 5.9 | 4.48 |
| Calcium | 0.9 | 0.037 |
| Phosphate | 0.7 | 0.36 |
| Sodium | 0.15 | 0.133 |
Sample collection and analysis: urine collection and measurement
All blood and sample collections were performed in an identical manner in Sweden and Poland. The 24-hour urine samples were collected from the metabolic cages into a bucket placed underneath the cage, containing 5 to 15 mL of 6N hydrochloric acid, in order to acidify samples and achieve a pH of below 3, which was necessary to maintain all oxalate soluble in the urine. After measurement of total urine volume, 3 mL samples extracted with charcoal were transferred to plastic tubes for future analysis. The 24 hour collections were taken 5 times during period P1, twice during P2, twice during P3, once at the end of period P4, once at the end of P5, and 3 times at the end of P6.
Blood samples were collected before the morning meal from jugular vein catheters on the same day as the 24-hr urine collections, during the respective experimental periods. Blood samples were collected into heparin tubes and centrifuged for 15 minutes at 3000 rpm, and blood plasma was separated into new tubes. Samples were stored at -20°C for further analysis. Creatinine concentration in blood plasma and urine was analyzed using a Mindray BS-130 analyzer. Total oxalate concentration in urine was measured using oxalate reagents (Kit 591D, Trinity Biotech, Ireland) according to the manufacturer’s instructions. Urinary Ca2+ was determined by means of an ion analyser (BioMaxima, Poland). The creatinine clearance was estimated based on plasma and urine creatinine concentration and as well as diuresis according to the following equation: CCr = (UCr x V/PCr.)/bwt and the results are presented as ml/min/kg b wt. Daily urinary excretion of creatinine, oxalate and Ca2+ was calculated based on daily urine volume and urine concentration.
Post-mortem analysis and histopathology
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Santry HP, Gillen DL, Lauderdale DS: Trends in bariatric surgical procedures. JAMA 2005 Oct 19; 294(15): 1909-1917.
2. Lieske JC, Kumar R, Collazo-Clavell ML: Nephrolithiasis after bariatric surgery for obesity. Semin Nephrol 2008 Mar; 28(2): 163-173.
3. Rao RS, Rao V, Kini S: Animal models in bariatric surgery – a review of the surgical techniques and postsurgical physiology. Obes Surg 2010 Sep; 20(9): 1293-1305.
4. Sinha MK, Collazo-Clavell ML, Rule A et al.: Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 2007 Jul; 72(1): 100-107.
5. Patel BN, Passman CM, Fernandez A et al.: Prevalence of hyperoxaluria after bariatric surgery. J Urol 2009 Jan; 181(1): 161-166.
6. Navarro-Díaz M, Serra A, Romero R et al.: Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 2006 Dec; 17(12 suppl. 3): S213-217.
7. Matlaga BR, Shore AD, Magnuson T et al.: Effect of gastric bypass surgery on kidney stone disease. J Urol 2009; 181: 2573-2577.
8. Whitson JM, Stackhouse GB, Stoller ML: Hyperoxaluria after modern bariatric surgery: case series and literature review. Int Urol Nephrol 2010 Jun; 42(2): 369-374.
9. Nelson WK, Houghton SG, Milliner DS et al.: Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 2005; 1: 481-485.
10. Ahmed MH, Byrne CD: Bariatric surgery and renal function: a precarious balance between benefit and harm. Nephrology, Dialysis, Transplantation 2010; 25(10): 3142-3147.
11. Navaneethan SD, Yehnert H, Moustarah F et al.: Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2009; 4: 1565-1574.
12. Liebman M, Costa G: Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol 2000 May; 163(5): 1565-1569.
13. Curhan GC, Willett WC, Knight EL, Stampfer MJ: Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med 2004 Apr 26; 164(8): 885-891.
14. Zingg U, Oertli D: Functional syndromes after surgery of the upper gastrointestinal tract. Therapeutische Umschau 2012; 69: 39-47.
15. Cartery C, Faguer S, Karras A et al.: Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol 2011 Aug; 6(8): 1895-1902.
16. Grases F, García-Ferragut L, Costa-Bauzá A: Study of the early stages of renal stone formation: experimental model using urothelium of pig urinary bladder. Urol Res 1996; 24(5): 305-311.
17. Khan SR: Animal models of kidney stone formation: an analysis. World J Urol 1997; 15(4): 236-243.
18. Meguid MM, Ramos EJ, Suzuki S et al.: A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg 2004 Jul-Aug; 8(5): 621-630.
19. O’Connor RC, Worcester EM, Evan AP et al.: Nephrolithiasis and nephrocalcinosis in rats with small bowel resection. Urol Res 2005 May; 33(2): 105-115.
20. Worcester EM, Chuang M, Laven B et al.: A new animal model of hyperoxaluria and nephrolithiasis in rats with small bowel resection. Urol Res 2005 Nov; 33(5): 380-382.
21. Khan SR, Glenton PA, Byer KJ: Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int 2006 Sep; 70(5): 914-923.
22. Chelikani PK, Shah IH, Taqi E et al.: Comparison of the effects of Roux-en-Y gastric bypass and ileal transposition surgeries on food intake, body weight, and circulating peptide YY concentrations in rats. Obes Surg 2010 Sep; 20(9): 1281-1288.
23. Gandarillas M, Bas F: The domestic pig (Sus scrofa domestica) as a model for evaluating nutritional and metabolic consequences of bariatric surgery practiced on morbid obese humans. Cien Inv Agr 2009; 36(2): 163-176.
24. Thomsen HS, Larsen S, Talner LB: Papillary morphology in adult human kidneys and in baby and adult pig kidneys. Eur Urol 1983; 9(3): 170-180.
25. USDA, 2000. Animal welfare information. Information resources on swine in biomedical research 1990-2000. Agricultural research service library. http://www.nal.usda.gov/awic/pubs/swine.htm#art.
26. Mandel NS, Henderson JD Jr, Hung LY et al.: A porcine model of calcium oxalate kidney stone disease. J Urol 2004 Mar; 171(3): 1301-1303.
27. Patel SR, Penniston KL, Iwicki L et al.: Dietary induction of long-term hyperoxaluria in the porcine model. J Endourol 2012 May; 26(5): 433-438.
28. Stanowski E, Paśnik K: Bariatric surgery – the current state of knowledge. Videosurgery and Other Miniinvasive Techniques 2008; 3(2): 71-86.
29. Penniston KL, Kaplon DM, Gould JC, Nakada SY: Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol 2009 Nov; 182(5): 2340-2346.
30. Maalouf NM, Tondapu P, Guth ES et al.: Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol 2010 Mar; 183(3): 1026-1030.
31. Currie A, Chetwood A, Ahmed AR: Bariatric surgery and renal function. Obes Surg 2011 Apr; 21(4): 528-539.
32. Kumar R, Lieske JC, Collazo-Clavell ML et al.: Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 2011 May; 149(5): 654-661.
33. Agrawal V, Shah A, Rice C et al.: Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol 2009 Sep; 5(9): 520-528.
34. Froeder L, Arasaki CH, Malheiros CA et al.: Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol 2012 Dec; 7(12): 2033-2040.
35. Duffey BG, Pedro RN, Makhlouf A et al.: Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg 2008 Jun; 206(3): 1145-1153.
36. Duffey BG, Alanee S, Pedro RN et al.: Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg 2010 Jul; 211(1): 8-15.
37. Harbottle L: Audit of nutritional and dietary outcomes of bariatric surgery patients. Obes Rev 2011; 12: 198-204.
38. Maier GW, Kreis ME, Zittel TT, Becker HD: Calcium regulation and bone mass loss after total gastrectomy in pigs. Ann Surg 1997 Feb; 225(2): 181-192.
39. Lehto-Axtelius D, Surve VV, Johnell O, Håkanson R: Effects of calcium deficiency and calcium supplementation on gastrectomy-induced osteopenia in the young male rat. Scand J Gastroenterol 2002 Mar; 37(3): 299-306.
40. Fakhouri F, Chauveau D, Touam M et al.: Crystals from fat. Acute oxalate nephropathy. Nephrol Dial Transplant 2002 Jul; 17(7): 1348-1350.
41. Puzio I, Kapica M, Filip R et al.: Fundectomy evokes elevated gastrin and lowered ghrelin serum levels accompanied by decrease in geometrical and mechanical properties of femora in rats. Bull Vet Inst Pulawy 2005; 49: 69-73.

