© Borgis - Postępy Nauk Medycznych 11/2015, s. 800-805
Dorota Jarzębicka, Joanna Sieczkowska, Grzegorz Oracz, *Jarosław Kierkuś
Ocena skuteczności i bezpieczeństwa terapii adalimumabem u dzieci z chorobą Leśniowskiego-Crohna
Evaluation of the efficacy and safety of adalimumab therapy in pediatric patients with Crohn’s disease
Department of Gastroenterology, Hepatology, Feeding Disorders and Pediatrics, The Children’s Memorial Health Institute, Warszawa
Head of Department: prof. Józef Ryżko, MD, PhD
Streszczenie
Wstęp. Choroba Crohna należy do grupy nieswoistych zapaleń jelit (IBD). Wczesna diagnoza i optymalne leczenie gwarantują pomyśle wyniki terapii, czyli uzyskanie remisji choroby. Wprowadzenie leków biologicznych do leczenia IBD zwiększyło efektywność uzyskiwania remisji choroby.
Cel pracy. Celem pracy była ocena skuteczności indukcji i utrzymania remisji choroby oraz bezpieczeństwa terapii adalimumabem u dzieci z chorobą Leśniowskiego-Crohna.
Materiał i metody. Badaniem objęto 9 pacjentów z chorobą Leśniowskiego-Crohna w średnim wieku 7,35 roku w chwili diagnozy. Wszyscy pacjenci leczeni byli w przeszłości IFX lub ADA. Do oceny aktywności choroby i skuteczności leczenia stosowano skalę PCDAI (Pediatric Crohn’s Disease Activity Index) oraz wartości badań laboratoryjnych: OB, CRP, hemoglobiny, hematokrytu w trakcie kwalifikacji do leczenia, po trzeciej dawce, a także po 6 i 12 miesiącach leczenia. Autorzy oceniali bezpieczeństwo terapii adalimumabem poprzez ocenę działań niepożądanych występujących podczas podawania leku i pomiędzy dawkami.
Wyniki. 8 chorych (89%) osiągnęło indukcję remisji, 1 (11%) pacjent nie odpowiedział na leczenie, przy czym jest to pacjent uprzednio leczony zarówno IFX, jak i ADA. W odniesieniu do działań niepożądanych (AE), autorzy nie zarejestrowali żadnych podczas podawania leku, a te, które miały miejsce między dawkami leku, nie różniły się od występujących w zdrowej populacji.
Wnioski. Terapia adalimumabem pozwala na indukcję i utrzymanie remisji choroby. Profil bezpieczeństwa stosowania adalimumabu w populacji pediatrycznej jest porównywalny z obserwowanym w populacji dorosłych.
Summary
Introduction. Crohn’s disease belongs to the group of inflammatory bowel disease (IBD). Prompt diagnosis and early optimal treatment warrants successful outcome, which is disease remission. Introduction of biological drugs for the treatment of IBD increased the efficiency of remission.
Aim. The aim of the study was to evaluate the effectiveness of induction and maintenance remission of the disease and safety of adalimumab therapy in pediatric patients with Crohn’s disease.
Material and methods. The study included 9 patients with Crohn’s disease with mean age 7.35 years at diagnosis. All patients were treated in the past with IFX or ADA. To assess the severity of the disease and the effectiveness of treatment authors used PCDAI scale (Pediatric Crohn’s Disease Activity Index) and value of laboratory tests – ESR, CRP, hemoglobine, hematocrite – during enrolment to therapy, after third dose and after 6 and 12 months of treatment. The authors assessed safety of adalimumab therapy by evaluation of adverse events occurring during the drug administration and between doses.
Results. 8 patients (89%) achieved induction of remission, 1 patient (11%) didn’t respond to the treatment, while this is the patient previously treated both IFX and ADA. Regard to adverse events (AE), the authors did not register any AE during administration of the drug, and those which occurred between doses of the drug were not different from those in a healthy population.
Conclusions. Adalimumab therapy allows to induction and maintenance remission. The safety profile of adalimumab in pediatric population is comparable with that seen in the adult population.

Introduction
Recently there has been a worldwide increase in the incidence of the Crohn’s disease in the adult and pediatric population (1, 2). The aim of the treatment of Crohn’s disease is to induce and then maintenance remission. In Poland treatment regimen is based on “step-up” strategy. It involves the use of drugs from the lowest level of the “treatment pyramid in IBD” (fig. 1) through gradual introduction drugs with increasing potency. Choosing the appropriate treatment depends on the severity of the disease, its extent or presence of the disease complications. Current treatment regimens according to the ECCO/ESPEGHAN guidelines, particularly in the newly diagnosed patients and those who are still in the maturation period, recommend the use of total enteral nutrition (EEN) for 6-8 weeks. In case of intolerance or lack of clinical improvement within 2 weeks of use EEN, corticosteroids in full dose for 2-4 weeks with subsequent, weekly, gradual dose reduction are recommended. In case of severe disease and poor prognosis, simultaneously immunosuppressive therapy with azathioprine or methotrexate, in order to maintain longer remission, is recommended. Biological treatment, in Crohn’s disease, stays at the top of the pyramid treatment (3). In Poland, for pediatric patients, Infliximab and Adalimumab are available. The indications for the therapy with anti-TNF are active, severe inflammation of the intestines and/or perianal lesions with the ineffectiveness of the drugs with less potency (1, 3-8). “Top-down” therapy – attempt to induce induction of remission, starting from the top of the pyramid treatment, in Poland is rarely used.
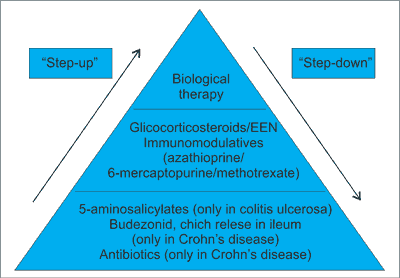
Fig. 1. Treatment pyramid in IBD.
The first approved biological drug, introduced for treatment inflammatory bowel disease, was Infliximab (IFX). It is a chimeric human-murine monoclonal immunoglobulin G (Ig) subclass G1. The drug is given intravenously at dose 5 mg/kg of body weight. It consists in 75% of human and 25% of murine components. The presence of murine components, potentailly can cause immunogenicity. Administration of each next doses of drug may result in production of antibodies against drug causing lower effectiveness of treatment and symptoms of intolerance of the drug. Numerous data from the literature describe adverse reactions associated with the administration of IFX. The most common are allergic reactions, with varying degrees of severity – from mild like redness of the skin or tachycardia, through serious – up to anaphylactic shock inclusive. Temporarily interruption or slowing infusion, with antihistamines usually causes resolution of symptoms. In some cases, however, it is necessary to discontinuation drug infusion. Because of these, searching for the new substance, constructed entirely of human aminoacid sequences was taken. Such drug is adalimumab (ADA). It is a recombinant, fully human monoclonal antibody with the immunomodulating activities. It is administered subcutaneously. It is used to induce and maintain disease remission (1, 2, 4, 9-11). Both drugs showed comparable efficacy in the induction of remission. The decision which drug will be use depends on the availability, patient preference and costs of the treatments. In Poland there is treatment program with infliximab for pediatric patients with Crohn’s disease paid by the National Health Fund, that is why adalimumab is rarely used, most common in case of intolerance or loss of response to infliximab. However, current ECCO/ESPEGHAN guidelines are putting both drugs equally. Moreover, subcutaneous administration of adalimumab creates the possibility of therapy at home, which is especially important for children, compared to IFX which is administered intravenously and patient must be hospitalized (3). As mentioned above, in Poland therapeutic program for treatment Crohn’s disease with adalimumab is still not available (despite the good performance of first-line therapy), that is why treatment with this preparation is possible only within hospital resources and limited to the hospital environment. In addition, an existing therapeutic program allows treatment with infliximab only severe Crohn’s disease, with PCDAI above 52 points. Recently, definition of the severity of Crohn’s disease in children has been changed. Currently disease with PCDAI above 40 points is considered to be severe and classifying the patient with PCDAI above 52 points indicates that achieving the expected effect of the treatment will be more difficult. Experts call for lowering the criteria for inclusion to the therapeutic program, it is known that due to the long-term course of the disease and its devastating impact, both on the body and psyche of children and young people, each delayed in treatment makes worse prognosis and makes it more difficult to achieve disease remission. However, it didn’t reflected for inclusion criteria to the therapeutic program.
Adalimumab is currently the only anti-TNF drug, which has a pediatric registration in all three therapeutic areas: rheumatology (juvenile idiopathic arthritis from 2 y.), dermatology (psoriasis in children and adolescents aged 4 y.) and gastroenterology (Crohn’s disease from 6 y.). No other TNF or biological drug with a different mechanism of action don’t have confirmed the efficacy and safety profile in the pediatric population as much as adalimumab.
Aim
The aim of the study was to evaluate the effectiveness of induction and maintenance remission of the disease and safety of adalimumab therapy in pediatric patients with Crohn’s disease.
Material and methods
Therapy with biological drugs is initiated from the 3 induction doses. Cut-off line for adalimumab dose is patient weight of 40 kg. For patients weighing less than 40 kg drug is administered in the regimen 80-40-20 mg/dose/every 2 weeks, patients weighing more than 40 kg respectively 160-80-40 mg/dose/every 2 weeks. It is recommended to administrate each subsequent doses at two-week intervals. When we can observe loss of response to a drug, in practice, decreasing the interval of administration on a weekly injection is possible. To assess the severity of disease and the effectiveness of treatment authors used a PCDAI scale (Pediatric Crohn’s Disease Activity Index) and value of laboratory tests – ESR, CRP, hemoglobine, hematocrite, during qualifications for the treatment, at 3 dose in order to assess the induction of remission, after 6 months of treatment and in 3 patients even after 12 months of treatment.
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Sherlock ME, Griffiths AM: Medical Therapy for Pediatric Inflammatory Bowel Disease. Curr Gastroenterol Rep 2012; 14: 166-173.
2. Magro F, Portela F: Management of Inflammatory Bowel Disease with Infliximab and Other Anti-Tumor Necrosis Factor Alpha Therapies. Biodrugs 2010; 24 (suppl. 1): 3-14.
3. Ruemmele FM, Veres G, Kolho KL et al.: Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. Journal of Crohn’s and Colitis 2014; 8: 1179-1207.
4. Song Y-N, Zheng P, Xiao J-H, Lu Z-J: Efficacy and safety of adalimumab for the Crohn’s disease: a systematic review and meta-analysis of published randomized placebo-controlled trias. Eur J Clin Pharmacol 2014; 70: 907-914.
5. Velayos FS, Sandborn WJ: Positioning Biologic Therapy for Crohn’s Disease and Ulcerative Colitis. Current Gastroenterology Reports 2007; 9: 521-527.
6. Assche GV, Vermeire S, Rutgeerts P: Optimizing Treatment of Inflammatory Bowel Diseases with Biologic Agents. Current Gastroenterology Reports 2008; 10: 591-596.
7. Kierkus J, Dadalski M, Szymanska E et al.: The impact of infliximab induction therapy on mucosal healing and clinical remission in Polish pediatric patients with moderate-to-severe Crohn’s disease. Eur J Gastroenterol Hepatol 2012; 24(5): 495-500.
8. Kierkus J, Dadalski M, Szymanska S et al.: Maintenance therapy with infliximab for paediatric Crohn’s disease: impact on clinical remission and mucosal healing in Polish paediatric patients with severe Crohn’s disease. Prz Gastroenterol 2012; 7(1): 26-30.
9. Navas-López VM, Blasco-Alonso J, Girón-Fernández-Crehuet F et al.: Efficacy and safety of adalimumab in the treatment of Crohn’s disease in children. Rev Esp Enferm Dig 2013; 105: 579-584.
10. Cozijnsen M, Duif V, Kokke F et al.: Adalimumab Therapy in Children With Crohn Disease Previously Treated With Infliximab. JPGN 2015; 60: 205-210.
11. Breda L, Del Torto M, De Sanctis S, Chiarelli F: Biologics in children’s autoimmune disorders: efficacy and safety. Eur J Pediatr 2011; 170: 157-167.
12. Rosenbach Y, Hartman C, Shapiro R et al.: Adalimumab Treatment in Children with Refractory Crohn’s Disease. Dig Dis Sci 2010; 55: 747-753.
13. Hyams J, Griffiths A, Markowitz J et al.: Safety and Efficacy of Adalimumab for Moderate to Severe Crohn’s Disease in Children. Gastroenterology 2012; 143: 365-374.
14. Dewint P, Hansen BE, Verhey E et al.: Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomized, double-blind, placebo controlled trial (ADAFI). Gut 2014; 63: 292-299.

