Department of Endocrinology, Centre of Postgraduate Medical Education, Bielański Hospital, Warszawa
Head of Department: prof. Wojciech Zgliczyński, MD, PhD
Medullary thyroid cancer (MTC) is a malignancy that originates from the neuroendocrine calcitonin-secreting parafolicullar C cells. According to the latest data it represents only about 1-2% of the thyroid cancers (1). Due to another origin than differentiated thyroid cancers groving from thyrocytes it has different biology and worse prognosis, accounting for 13.4% of deaths caused by thyroid cancers (2). Approximately 25% of the MTC are the forms of hereditary syndromes associated with type 2 multiple endocrine neoplasia (MEN 2) (1). MTC has a high tendency to metastasize both by lymphatic system (to the cervical lymph nodes, mediastinum and pulmonary hila) and by blood (most often to the liver, lungs, bones, rarely to the brain, breast and skin). In 81% of palpable tumours lymph node matastasis is found on the side of the tumour, and in 44% – at the opposite side (3). Even if at the time of diagnosis the distant metastases are not found, the recurrence rate after total thyroidectomy with lymphadenectomy is 50% (4).
Ultrasonography of the neck plays a fundamental role in the diagnosis of the primary tumour. Computed tomography (CT) and magnetic resonance imaging (MRI) are used mainly to assess tumour infiltration of the surrounding muscles, trachea, larynx, oesophagus, retro-oesophageal space and mediastinum.
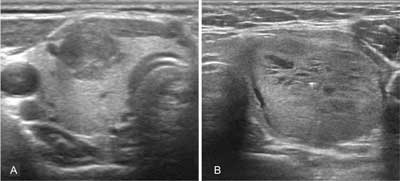
Medullary cancer observed in ultrasonographic imaging is not significantly different from other thyroid cancers. As the tumour originates from parafollicular C cells, which are mainly located in the upper and central parts of the lobes, it is often found there. In heritable forms, the lesions are frequently multifocal, and may be present in both lobes (1). Ultrasound features indicating the malignancy of the thyroid tumour is a solid structure, hypoechogenicity, enhanced central flow, irregular margin, anterior-posterior dimension greater than the transverse one and internal calcifications (especially microcalcifications) (fig. 1A) (8, 9). Compared to the papillary cancer, however, MTC more often exhibitis the characteristics of benign lesions such as oval shape, homogeneous structure, cystic degenerative lesions or smooth well-defined margins (fig. 1B) (10). According to some authors, the morphology of up to 1/3 of the medullary cancers is not suspected in ultrasound imaging (9, 10). In the elastographic examination, MTC often does not have a typical for cancer “hard” character also (11). Hence, many experts call for the introduction of routine calcitonin determination in the diagnosis of thyroid focal lesions.
Ultrasound imaging is the basis for the classification of thyroid tumours for fine needle aspiration biopsy (FNAB), which is the golden standard in the diagnosis of thyroid cancer. FNAB sensitivity in the diagnosis of MTC is about 44-63% (12, 13). Medullary thyroid cancer may be misdiagnosed as follicular tumour, parathyroid tumour, poorly differentiated cancer of unknown aetiology or (rarely) anaplastic cancer. Immunocytochemical detection of calcitonin, calcitonin gene-related peptide (CGRP), chromogranin A, CEA or neuron-specific enolase (NSE), as well as calcitonin measurement in fine-needle aspiration biopsy wash-out fluid, significantly increase the diagnostic value of FNAB (1).
In the recurrence of MTC, the most important prognostic factors are early diagnosis, location of the tumour foci and proper qualification of patients for further treatment. In monitoring a patient after the surgery, it is essential to determine the concentrations of calcitonin and CEA periodically. The first test is recommended after 3 months (1). It shall not be performed earlier than 2-3 months after the surgery, due to the possibility of increased levels of calcitonin during wound healing and long half-life of calcitonin and CEA. Further measurements should be made depending on the results: if the concentrations are undetectable – every 6 months in the first year and then every 12 months; if they are detectable – at least every 6 months (1). In the case of undetectable or normal basal concentrations of calcitonin, the ultrasound of the neck should be performed and the stimulation test with pentagastrin or calcium (15) shall be considered.
In the case of local recurrence, reoperation is the only treatment that gives a chance for long-term remission or even curing the patient (16).
Imagining of both local recurrence as well as distant metastases of MTC is often a big challenge for the clinician. None of the currently available methods is sufficiently sensitive in the detection of local recurrence or metastatic lesions to be used alone. Even the combination of various diagnostic procedures allows for location of the recurrent tumour in approximately 40% of patients only (17).
The lymph nodes are the most common site of MTC metastasis. At the time of diagnosis lymph node metastases are found in 75-81% of patients and the distant ones – in up to 13% (3, 18, 19).
In patients with calcitonin concentration of up to 150 pg/ml, the first-line examination is the ultrasound of the neck, since in these cases local recurrence or metastasis to the neck lymph nodes are most common (1). The lymph nodes with metastatic lesions are usually oval or round in shape (the ratio of the long to short axis of node is < 1.5), with blurred hilum, increased heterogeneous echogenicity, contain cystic degenerative lesions or calcifications and have chaotic blood flow in Doppler imaging. The sensitivity of ultrasound is 46-88%, similar to CT (42-86%) and MRI of the neck (38-91%), which are, however, complementary, as they allow to visualize the nodes inaccessible by ultrasound, located in the retro-tracheal as well as in the para- and retro-oesophageal space (20-25). An important role in the diagnosis of nodal metastases, especially when small nodes with no obvious morphological changes are found, plays the FNAB with the assessment of calcitonin concentration in the biopsy needle wash-out fluid (26).
1. Wells SA Jr, Asa SL, Dralle H et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25(6): 567-610.
2. Carling T, Udelsman R: Thyroid tumours. [In:] DeVita VT, Hellman S, Rosenberg SA (eds.): Cancer: Principles and Practice of Oncology. Lippincott Williams & Wilkins, Philadelphia 2008: 1663-1682.
3. Moley JF, DeBenedetti MK: Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 1999; 229(6): 880-887.
4. Sippel RS, Kunnimalaiyaan M, Chen H: Current management of medullary thyroid cancer. Oncologist 2008; 13(5): 539-547.
5. Cohen R, Campos JM, Salaun C et al.: Preoperative calcitonin levels are predictive of tumour size and postoperative calcitonin normalization in medullary thyroid carcinoma. Groupe d’Etudes des Tumeurs a Calcitonine (GETC). J Clin Endocrinol Metab 2000; 85(2): 919-922.
6. Machens A, Dralle H: Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010; 95(6): 2655-2663.
7. Modigliani E, Cohen R, Campos JM et al.: Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 1998; 48: 265-273.
8. Woliński K, Rewaj-Łosyk M, Ruchała M: Sonographic features of medullary thyroid carcinomas – a systematic review and meta-analysis. Endokrynol Pol 2014; 65(4): 314-318.
9. Choi N, Moon WJ, Lee JH et al.: Ultrasonographic findings of medullary thyroid cancer: differences according to tumour size and correlation with fine needle aspiration results. Acta Radiol 2011; 52(3): 312-316.
10. Trimboli P, Giovanella L, Valabrega S et al.: Ultrasound features of medullary thyroid carcinoma correlate with cancer aggressiveness: a retrospective multicenter study. J Exp Clin Cancer Res 2014; 33(1): 87.
11. Andrioli M, Trimboli P, Amendola S et al.: Elastographic presentation of medullary thyroid carcinoma. Endocrine 2014; 45(1): 153-155.
12. Essig GF Jr, Porter K, Schneider D et al.: Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocr Pract 2013; 19(6): 920-927.
13. Bugalho MJ, Santos JR, Sobrinho L: Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol 2005; 91(1): 56-60.
14. Scollo C, Baudin E, Travagli JP et al.: Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2003; 88(5): 2070-2075.
15. Jarząb B, Sporny S, Lange D et al.: Diagnostyka i leczenie raka tarczycy – rekomendacje polskie. Endokrynol Pol 2010; 61(5): 518-568.
16. Fialkowski E, DeBenedetti M, Moley J: Long-term outcome of reoperations for medullary thyroid carcinoma. World J Surg 2008; 32(5): 754-765.
17. Rufini V, Castaldi P, Treglia G et al.: Nuclear medicine procedures in the diagnosis and therapy of medullary thyroid carcinoma. Biomed Pharmacother 2008; 62(3): 139-146.
18. Weber T, Schilling T, Frank-Raue K et al.: Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery 2001; 130(6): 1044-1049.
19. Ganeshan D, Paulson E, Duran C et al.: Current update on medullary thyroid carcinoma. AJR Am J Roentgenol 2013; 201(6): W867-W876.
20. Raue F, Winter J, Frank-Raue K et al.: Diagnostic procedure before reoperation in patients with medullary thyroid carcinoma. Horm Metab Res Suppl 1989; 21: 31-34.
21. Dörr U, Würstlin S, Frank-Raue K et al.: Somatostatin receptor scintigraphy and magnetic resonance imaging in recurrent medullary thyroid carcinoma: a comparative study. Horm Metab Res Suppl 1993; 27: 48-55.
22. Wang Q, Takashima S, Fukuda H et al.: Detection of medullary thyroid carcinoma and regional lymph node metastases by magnetic resonance imaging. Arch Otolaryngol Head Neck Surg 1999; 125: 842-848.
23. Kebebew E, Kikuchi S, Duh QY, Clark OH: Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg 2000; 135(8): 895-901.
24. Giraudet AL, Vanel D, Leboulleux S et al.: Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J Clin Endocrinol Metab 2007; 92(11): 4185-4190.
25. Sesti A, Mayerhoefer M, Weber M et al.: Relevance of calcitonin cut-off in the follow-up of medullary thyroid carcinoma for conventional imaging and 18-fluorine-fluorodihydroxyphenylalanine PET. Anticancer Res 2014; 34(11): 6647-6654.
26. Boi F, Maurelli I, Pinna G et al.: Calcitonin measurement in wash-out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 2007; 92(6): 2115-2118.
27. Nofech-Mozes S, Mackenzie R, Kahn HJ et al.: Breast metastasis by medullary thyroid carcinoma detected by FDG positron emission tomography. Ann Diagn Pathol 2008; 12(1): 67-71.
28. Nashed C, Sakpal SV, Cherneykin S, Chamberlain RS: Medullary thyroid carcinoma metastatic to skin. J Cutan Pathol 2010; 37(12): 1237-1240.
29. Tung WS, Vesely TM, Moley JF: Laparoscopic detection of hepatic metastases in patients with residual or recurrent medullary thyroid cancer. Surgery 1995; 118: 1024-1029.
30. Leclère J, Sidibè S, Lassau N et al.: Ultrasonographic aspects of hepatic metastases of thyroid medullary cancers. J Radiol 1996; 77(2): 99-103.
31. Van Beers B, Pringot J, Defalque D: Hepatic metastases in medullary thyroid carcinoma: possible pitfall with MR imaging. Eur J Radiol 1990; 11(2): 107-109.
32. Hung WW, Wang CS, Tsai KB et al.: Medullary thyroid carcinoma with poor differentiation and atypical radiographic pattern of metastasis. Pathol Int 2009; 59(9): 660-663.
33. Mazoyer G, Cordier JF, Zabern JM et al.: Pulmonary metastases in medullary cancers of the thyroid. Study of 4 cases. Originality of the lymphangitic form with amyloid stroma. Rev Mal Respir 1986; 3(3): 139-143.
34. Miralliè E, Vuillez JP, Bardet S et al.: High frequency of bone/bone marrow involvement in advanced medullary thyroid cancer. J Clin Endocrinol Metab 2005 Feb; 90(2): 779-788.
35. Skoura E: Depicting medullary thyroid cancer recurrence: the past and the future of nuclear medicine imaging. Int J Endocrinol Metab 2013; 11(4): e8156.
36. Maiza JC, Grunenwald S, Otal P et al.: Use of 131I-MIBG therapy in MIBG-positive metastatic medullary thyroid carcinoma. Thyroid 2012; 22: 654-655.
37. Baulieu JL, Guilloteau D, Delisle MJ et al.: Radioiodinated meta-iodobenzylguanidine uptake in medullary thyroid cancer. A French cooperative study. Cancer 1987; 60(9): 2189-2194.
38. Verga U, Muratori F, Di Sacco G et al.: The role of radiopharmaceuticals MIBG and (V) DMSA in the diagnosis of medullary thyroid carcinoma. Henry Ford Hosp Med J 1989; 37(3-4): 175-177.
39. Clarke S, Lazarus C, Maisey M: Experience in imaging medullary thyroid carcinoma using 99mTc (V)-dimercaptosuccinic acid (DMSA). Henry Ford Hosp Med J 1989; 37(3-4): 167-168.
40. Arslan N, Ilgan S, Yuksel D et al.: Comparison of In-111 octreotide and Tc-99m (V) DMSA scintigraphy in the detection of medullary thyroid tumor foci in patients with elevated levels of tumor markers after surgery. Clin Nucl Med 2001; 26(8): 683-688.
41. Baudin E, Lumbroso J, Schlumberger M et al.: Comparison of octreotide scintigraphy and conventional imaging in medullary thyroid carcinoma. J Nucl Med 1996; 37(6): 912-916.
42. Kaltsas G, Korbonits M, Heintz E et al.: Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. J Clin Endocrinol Metab 2001; 86(2): 895-902.
43. Behr TM, Gratz S, Markus PM et al.: Anti-carcinoembryonic antigen antibodies versus somatostatin analogs in the detection of metastatic medullarythyroid carcinoma: are carcinoembryonic antigen and somatostatin receptor expression prognostic factors? Cancer 1997; 80 (12 suppl.): 2436-2457.
44. Decristoforo C, Mather SJ, Cholewinski W et al.: 99mTc-EDDA/HYNIC-TOC: a new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours; first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur J Nucl Med 2000; 27(9): 1318-1325.
45. Parisella M, D’Alessandria C, van de Bossche B et al.: 99mTc-EDDA/HYNIC-TOC in the management of medullary thyroid carcinoma. Cancer Biother Radiopharm 2004; 19(2): 211-217.
46. Czepczyński R, Parisella MG, Kosowicz J et al.: Somatostatin receptor scintigraphy using 99mTc-EDDA/HYNIC-TOC in patients with medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2007; 34(10): 1635-1645.
47. Sager S, Kabasakal L, Ocak M et al.: Clinical value of technetium-99m--labeled octreotide scintigraphy in local recurrent or metastatic medullary thyroid cancers: a comparison of lesions with 18F-FDG-PET and MIBI images. Nucl Med Commun 2013; 34(12): 1190-1195.
48. Sandrock D, Blossey HC, Steinroeder M, Munz DL: Contribution of different scintigraphic techniques to the management of medullary thyroid carcinoma. Henry Ford Hosp Med J 1989; 37(3-4): 173-174.
49. Troncone L, Rufini V, De Rosa G, Testa A: Diagnostic and therapeutic potential of new radiopharmaceutical agents in medullary thyroid carcinoma. Henry Ford Hosp Med J 1989; 37(3-4): 178-184.
50. Ambrosini V, Tomassetti P, Franchi R, Fanti S: Imaging of NETs with PET radiopharmaceuticals. Q J Nucl Med Mol Imaging 2010; 54(1): 16-23.
51. Treglia G, Rufini V, Salvatori M et al.: PET Imaging in Recurrent Medullary Thyroid Carcinoma. Int J Mol Imaging 2012; 2012: 324686.
52. Szakáll S Jr, Esik O, Bajzik G et al.: 18F-FDG PET detection of lymph node metastases in medullary thyroid carcinoma. J Nucl Med 2002; 43(1): 66-71.
53. Rubello D, Rampin L, Nanni C et al.: The role of 18F-FDG PET/CT in detecting metastatic deposits of recurrent medullary thyroid carcinoma: a prospective study. Eur J Surg Oncol 2008; 34(5): 581-586.
54. Beuthien-Baumann B, Strumpf A, Zessin J et al.: Diagnostic impact of PET with 18F-FDG, 18F-DOPA and 3-O-methyl-6-[18F]fluoro-DOPA in recurrent or metastatic medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2007; 34(10): 1604-1609.
55. Treglia G, Castaldi P, Villani MF et al.: Comparison of 18F-DOPA, 18F-FDG and 68Ga-somatostatin analogue PET/CT in patients with recurrent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2012 Apr; 39(4): 569-580.
56. Skoura E, Rondogianni P, Alevizaki M et al.: Role of [(18)F]FDG-PET/CT in the detection of occult recurrent medullary thyroid cancer. Nucl Med Commun 2010; 31(6): 567-575.
57. Sesti A, Mayerhoefer M, Weber M et al.: Relevance of calcitonin cut-off in the follow-up of medullary thyroid carcinoma for conventional imaging and 18-fluorine-fluorodihydroxyphenylalanine PET. Anticancer Res 2014 Nov; 34(11): 6647-6654.
58. Marzola MC, Pelizzo MR, Ferdeghini M et al.: Dual PET/CT with 18F-DOPA and 18F-FDG in metastatic medullary thyroid carcinoma and rapidly increasing calcitonin levels: comparison with conventional imaging. Eur J Surg Oncol 2010; 36(4): 414-421.
59. Treglia G, Villani MF, Giordano A, Rufini V: Detection rate of recurrent medullary thyroid carcinoma using fluorine-18 fluorodeoxyglucose positron emission tomography: a meta-analysis. Endocrine 2012; 42(3): 535-545.
60. Luster M, Karges W, Zeich K et al.: Clinical value of 18-fluorine-fluorodihydroxyphenylalanine positron emission tomography/computed tomography in the follow-up of medullary thyroid carcinoma. Thyroid 2010; 20(5): 527-533.
61. Conry BG, Papathanasiou ND, Prakash V et al.: Comparison of 68Ga-DOTATATE and (18)F-fluorodeoxyglucose PET/CT in the detection of recurrent medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2010; 37(1): 49-57.
62. Verburg FA, Anlauf M, Mottaghy FM, Karges W: Somatostatin receptor imaging-guided pasireotide therapy in medullary thyroid cancer with ectopic adrenocorticotropin production. Clin Nucl Med 2015; 40(1): e83-e84.