*Agnieszka Staniewska
Safety of treatment and dietary supplementation with diosmin in daily doses up to 2000 mg a day
Specialist Medical Practice DAGDERM, Warsaw, Poland
Summary
Introduction. Diosmin is used to treat and alleviate the symptoms of venous insufficiency and haemorrhoids. Diosmin-containing medicinal products have been available in Poland since the 1990s with the status of drugs available on prescription in the form of flavonoid fraction containing diosmin, as a form subjected to micronisation or nonmicronised. For several years now, diosmin has been present on the pharmaceutical market in the form of over-the-counter medicines (OTC), and dietary supplements containing diosmin are available not only in pharmacies, but also in other outlets. Great availability of diosmin raises questions about the safety of a treatment without medical or pharmacist’s supervision. The safety of diosmin use in a daily dose of 2000 mg was confirmed in a double-blind, controlled placebo, randomised, three-arm and parallel, phase III to evaluate the efficacy and safety of diosmin administered for 4 months in the treatment of cellulite.
Aim. The aim of the study was to observe the efficacy and safety of micronised diosmin therapy used in a daily dose of 1000 and 2000 mg for a period of four months.
Material and methods. The trial involved 327 patients aged 18 to 55 (mean 37.28, standard deviation of 8.587) meeting the inclusion criteria and not meeting the exclusion criteria. The safety analysis includes and shows in a table the measured parameters immediately prior to the first dose, after 2 and 4 months of therapy.
Results. No statistically significant deviations from the standard condition in the following parameters present: complete blood count with differential, fibrinogen concentrations, alanine and aspartate transaminase concentration, urea and creatinine concentration, urinalysis. The therapy did not affect the value of systolic and diastolic blood pressure. No significant adverse actions in the studied groups.
Conclusions. The results of a clinical trial cited in this work support the use of micronised diosmin in doses up to 2000 mg per day.
INTRODUCtION
Diosmin is used in the treatment and alleviation of the symptoms of venous insufficiency and haemorrhoids. Diosmin-containing medicinal products have been available in Poland since the 1990s as prescription-only medicinal products. These products are present in the form of diosmin-containing flavonoid fraction, in the form subject to micronisation or non-micronised. There has been diosmin on the pharmaceutical market for several years now, which takes the form of OTC medications, while diosmin-containing dietary supplements are available not only at chemist’s but also other points, such as grocer’s and even pharmacies or petrol stations. In accordance with the observations and the results of many research on the substance, diosmin has anti-inflammatory operation being the result of inhibiting the excretion of prostaglandin (1-4), it increases the oncotic pressure of the lymph thus having an influence on reducing lymphoedema and enhancing flow in the lymphatic system (2, 5, 6). Diosmin also shows protective operation in the endothelium wall limiting its permeability (7). An important action of diosmin is increasing the vein wall tone, thus improving the return of blood from the venous system of lower extremities (1, 5, 8). Owing to such action, what is obtained is alleviating the concomitant symptoms and ailments of chronic venous insufficiency, such as contractions and pain in the calf, oedema, the feeling of heavy legs (9). Numerous research and clinical practice have shown that the most therapeutically effective form of diosmin is a cleansed flavonoid fraction subject to micronisation, which increases the absorption of the medicine (1, 6, 9). This is the form that the majority of preparations present on the pharmaceutical market take, while one should draw attention to the fact that these are products of various status, i.e. both medications and dietary supplements. Medications are products approved for marketing in Poland by the Office for Medicinal Products Registration and the product has a Summary of Product Characteristics available for the doctors and the patient, which contains detailed information essential for proper use and the course of treatment, information on the safety of therapy, recommended doses, etc. The patient can find information there on the fact that the treatment of chronic venous insufficiency involves usually micronised diosmin dose up to 1000 mg a day or 500 mg twice a day (9). In the case of treating haemorrhoid-related problems, the dose is higher and amounts to 3000 mg a day for 4 days, and then 2000 mg a day for the next 3 days. Administering diosmin in daily doses exceeding 1000 mg is time-limited up to 7 days. The dose of 1000 mg is usually applied in chronic therapy. In the case of dietary supplements, even in the case their composition is identical with the medicinal product, there are no rules of the pharmaceutical law applied. These products are subject to the requirements of the Food Law, among which special attention should be paid to the regulations concerning labelling, presentation and advertising. Pursuant to the binding law (Regulation 1163/2011) a dietary supplement marketed must contain the following data concerning its composition and use:
– names of nutrient categories or substances characterising the product or indication of their properties,
– recommended amount of product to be eaten a day,
– warning on not exceeding the recommended amount of product to be eaten a day,
– information that dietary supplements shall not be used as a substitute for a varied diet.
Diosmin-containing products are recommended to patients by doctors, pharmacists as well as used by patients during self-treatment on the basis of previous experience with that substance or on the basis of advertisements and information available in the media or the word-of-mouth marketing. It should be presumed that part of the users of that substance do it because they care about their healthy way of nutrition and supplement their diet with flavonoids, including ones with diosmin, owing to their proven or assumed good impact on our health. The boundary between traditional terms patient and consumer is thus fuzzy, and one should picture a person that should be called a patient in the case they take diosmin for medicinal purposes when recommended by a doctor, pharmacist or even in self-treatment, at the same time being a consumer of diosmin-containing food for dietary reasons. Therefore, people taking diosmin fall out of the traditional differentiation between the terms patient and consumer, that is the reason why the author uses these words interchangeably in the work. A similar obstacle is observed in the case of naming taking the preparations, i.e. in the case of using a product for medical reasons it is called therapy, treatment, in the case of dietary supplements it seems reasonable to call it eating, consumption. The issue of dosing is additionally complicated by the fact that since the 1990s, micronised diosmin has been available in doses of 500 mg in one tablet (also 450 mg diosmin and 50 mg hesperidin) or 600 mg in the case of non-micronised diosmin and standard dose is 2 tablets a day. At present, there are preparations available which contain 1000 mg to be administered once a day. This may result in a situation when patients are confused and may take diosmin in various doses, usually higher than recommended. It should also be noted that – in accordance with the reports from various clinical trials and the available publications, using diosmin in a dose higher than the one listed in the Summary of Product Characteristics, one may expect higher effectiveness as regards lowering the venous insufficiency-related complaints (5, 10-12), and additionally a beneficial impact of diosmin on the course of other diseases was described: diabetic retinopathy (13), restless legs syndrome, and even limiting the number of lung metastases in the case of melanoma (14, 15) and other neoplasms (16). High doses of diosmin may be thus taken wilfully. Should a thesis be added that part of patients may take simultaneously various diosmin-containing products due to various indications and various levels of recommendation, it forms the basis to ask a question about the safety of such a scheme of therapy and diet with the application of diosmin in doses exceeding 1000 mg a day.
Clinical trial results, the aim of which was to determine the effectiveness and confirm the safety of applying diosmin doses of 1000 and 2000 mg a day in chronic therapy among patients suffering from telangiectasia of lower extremities and subcutaneous tissue degeneration prove an important material to assess the therapy. Safety assessment was performed on the basis of a double blind, controlled placebo, randomised, three-arm parallel, study of the 3rd stage assessing the safety and efficacy of diosmin administered for the past 4 months in treating spider veins and fibrotic subcutaneous tissue degeneration, commonly known as cellulite. Verification of treatment safety was a secondary goal of the clinical trial indicated in the protocol.
AIM
The aim of the study was to confirm the safety of therapy and dietary supplementation with the use of diosmin at daily doses up to 2000 mg in up to four months. Safety has been assessed on the basis ofA phase III, double-blind, placebo-controlled, randomized, three-arm parallel, study assessing efficacy and safety of 4 months of diosmin administration for the treatment of lower extremity teleangiectasias and gynoid lipodystrophy (edematous fibrosclerotic panniculopathy commonly known as cellulite). Safety study treatment was secondary, as indicated in the protocol objective of the clinical trial.
MATERIAL AND METHODS
The protocol and all the research centres were approved by the Bioethics Committee at the Regional Medical Chamber in Warsaw, and all the researchers had the knowledge and experience as well as had been trained as regards the manner of running and documenting the results of clinical trials.
Safety-essential, binding inclusion criteria in the trial include to be a female, age ≥ 18 to ≤ 55, body mass index (BMI) 18.50-34.99 kg/m2, minimum body mass – 50 kg, the use of at least one effective contraception method or abandoning sex life within the trial, menstrual cycle within the range of 21 to 35 days and negative pregnancy test. Another important declaratory criterion is keeping the usual dietary habits, abstaining from making any significant changes in lifestyle. 327 patients were qualified for the trial and randomised. Patients included for the trial were aged 18-55, median amounting to 38, body mass within the range of 50-100 kg, median amounting to 66 kg. All the patients were the representatives of the Caucasian race, none of patients declared alcohol consumption every day. The patients did not smoke tobacco or take other drugs. Exclusion criteria essential for safety reasons and excluding from the trial include thrombosis in history, documented autoimmunological disorders, such as systemic lupus erythematosus, rheumatoid arthritis, lymphatic disorders, clotting disorders requiring one to use anticoagulants as well as heart failure, asthma, bronchitis, hypertension, diabetes of any type, existent or past diseases such as acute or chronic circulatory system disease, respiratory tract diseases, haematopoietic system diseases, diseases of the alimentary canal, urinary tract, hormone system, nervous system, musculoskeletal system, which may have an impact on the trial in the opinion of the performer, present alimentary canal diseases, diseases of the liver or kidneys, which may have an impact on the trial in the opinion of the performer, abnormal basal laboratory tests results, chronic medication application that may lead to disturbing the balance of fluids and/or blood circulation (e.g. diuretics, steroids, antidepressants, non-steroid anti-inflammatory drugs and others) which may have an impact on the trial in the opinion of the performer. Recruiting patients also excluded hormone contraception used for a period over six months, smoking tobacco, drinking alcohol in the amount exceeding two standard units a day and the use of any amounts of other drugs.
Patients were randomly grouped in the following groups taking placebo and/or medicine in the following configurations: A group – placebo (two tablets), twice a day, B group – 1000 mg diosmin once a day (two tablets 500 mg) and placebo (two tablets), once a day, and C group – 1000 mg diosmin (two tablets 500 mg twice a day, 2000 mg diosmin in total). Patients covered by the trial took the medicine and placebo for the period of 120 days. Final points as regards safety issues (with reference to basal points) were the following, in accordance with the protocol: laboratory tests results in haematology and biochemistry, urinalysis and checking the value of blood pressure as vital signs. Tests and measurements were performed during visit 1a (day 0), visit 2 (day 60 ± 7 days) and visit 3 (day 120 ± 7 days).
All the static analyses were conducted with the application of the “R” programme, version 3.2.0. Tables and data were generated in the Microsoft Word® programme.
The trial was conducted under project number POIG.01.04.00-04-200/12 entitled: “Implementing to the clinical practice in Poland and Europe an innovative medication in cellulite therapy” carried out by the company Alio Medica Sp. z o.o., project implemented under operation entitled: Support for targeted projects, Innovative Economy Operational Programme for the years 2007-2013.
Consent of the President of the Office for Medicinal Products, Medical Devices and Biocidal Products Registration was obtained to carry out clinical trial number UR/DBL/D/019/2014 and a positive opinion of the Bioethics Committee at the Regional Medical Chamber in Warsaw was obtained to carry out trial number KB/912/13.
RESULTS
The trial showed that as regards the efficacy (primary final point), there were no statistically essential differences between the group taking diosmin in the daily dose of 2000 mg and the group using placebo (p = 0.961) in the field of the following parameters: average values in the photonumeric Cellulite Severity Scale (CSS), the application of diosmin had no significant impact on the course of lipodystrophy. Statistically significant (p = 0.017) impact of diosmin on change in the length of the subcutaneous tissue stroma in the start and final point of treatment was shown in the case of the group taking diosmin in daily dose amounting to 2 g vs. placebo, which constitutes an interesting fact that may be the basis for further research as regards the efficacy of diosmin in dermatology and aesthetic medicine. In the case of issue previously mentioned, namely the assessment of safety of using diosmin, what proves essential are the results of physiological parameters and laboratory parameters obtained in the described research sample. Final results took into consideration data obtained from 327 patients. Safety analysis took into account and presented in the form of a table the results of measured parameters performed during visits 1, 2 and 3, namely directly prior to taking the first dose of diosmin, after 2 and 4 months. None of the measured parameters showed after 2 and 4 months of therapy any statistically significant (p ≥ 0.5) changes in biochemical parameters of the blood and urine as well as arterial blood pressure.
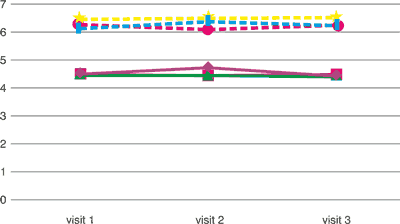
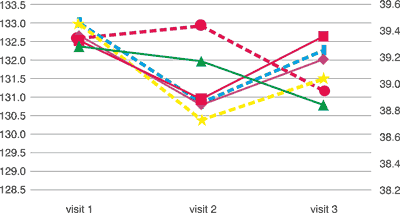
The studied group included no statistically significant deviations in testing the level of erythrocytes and leukocytes, both in the group taking placebo and in groups taking diosmin in the dose amounting to 1000 mg a day and 2000 mg a day after 2 and 4 months of therapy (tab. 1). There were no significant deviations observed in the image of blood smear and the number of platelets in the studied groups during subsequent visits. Haemoglobin and haematocrit concentration among the patients were within the range of standard values and small deviations in parameters were of no statistical significance (tab. 2). Diosmin used in the period of 4 months in the dose of 2000 mg a day had no significant impact on the image of complete blood count, all the parameters fell within the range of standard values.
Tab. 1. Erythrocytes and leukocytes in the tried groups of patients erythrocytes (millions/mm3; T/L] and leukocytes (thousands/mm3; g/L]
 |
| | visit 1 | visit 2 | visit 3 |
 erythrocytes [T/L] erythrocytes [T/L] | 4.493 | 4.723 | 4.422 |
 erythrocytes [T/L] erythrocytes [T/L] | 4.482 | 4.438 | 4.481 |
 erythrocytes [T/L] erythrocytes [T/L] | 4.444 | 4.442 | 4.394 |
 leukocytes [G/L] leukocytes [G/L] | 6.428 | 6.486 | 6.497 |
 leukocytes [G/L] leukocytes [G/L] | 6.125 | 6.363 | 6.236 |
 leukocytes [G/L] leukocytes [G/L] | 6.269 | 6.099 | 6.249 |
Tab. 2. Concentration of haemoglobin and haematocrit among the tried groups haemoglobin (g/l) and haematocrit (%)
 |
| | visit 1 | visit 2 | visit 3 |
 haemoglobin [g/L] haemoglobin [g/L] | 132.68 | 130.8 | 132.03 |
 haemoglobin [g/L] haemoglobin [g/L] | 132.54 | 130.96 | 132.65 |
 haemoglobin [g/L] haemoglobin [g/L] | 132.38 | 131.98 | 130.8 |
 haematoctrit [%] haematoctrit [%] | 39.453 | 38.739 | 39.044 |
 haematoctrit [%] haematoctrit [%] | 39.468 | 38.857 | 39.256 |
 haematoctrit [%] haematoctrit [%] | 39.346 | 39.442 | 38.950 |
Among the patients subject to the trial, the concentration of fibrinogen in serum was monitored during subsequent visits. It was confirmed that fibrinogen concentration during therapy underwent small changes, being at normal values and after 4 months of therapy slightly lower in the group taking diosmin in the dose of 2000 mg a day as compared to the group taking 1000 mg diosmin a day and the group taking placebo, which was of no statistical significance (tab. 3).
Tab. 3. Concentration of fibrinogen in the tried groups of patients
 |
| | visit 1 | visit 2 | visit 3 |
 placebo placebo | 303.848 | 302.517 | 292.320 |
 1000 mg 1000 mg | 311.291 | 305.600 | 308.714 |
 2000 mg 2000 mg | 305.282 | 285.593 | 296.297 |
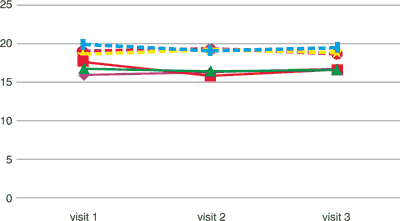
As regards biochemical parameters, in the studied groups of patients, it was confirmed that micronised diosmin therapy in the dose of 2000 mg a day had no statistically significant impact on the value of alanine and aspartate transaminase, and the value of hepatic enzymes fell within the standard range during the four-month therapy (tab. 4).
Tab. 4. Concentration of alanine transaminase and aspartate in the tried groups. Alanine transaminase and aspartate transaminase (units/litr, U/L)
 |
| | visit 1 | visit 2 | visit 3 |
 alanine transaminase [U/L] alanine transaminase [U/L] | 15.94 | 16.29 | 16.73 |
 alanine transaminase [U/L] alanine transaminase [U/L] | 17.60 | 15.82 | 16.64 |
 alanine transaminase [U/L] alanine transaminase [U/L] | 16.75 | 16.40 | 16.59 |
 aspartate transaminase [U/L] aspartate transaminase [U/L] | 18.54 | 19.12 | 18.80 |
 aspartate transaminase [U/L] aspartate transaminase [U/L] | 19.87 | 19.06 | 19.42 |
 aspartate transaminase [U/L] aspartate transaminase [U/L] | 18.87 | 19.30 | 18.74 |
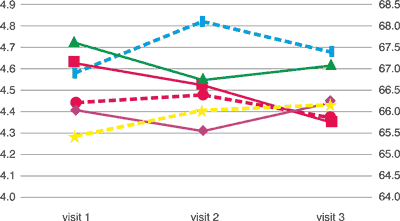
Also urea and creatinin concentration showed no deviations from the correct condition in all the examined groups during the trial (tab. 5).
Tab. 5. Concentration of urea and creatinine in the tried groups of patients. Urea (mmol/L) and creatinine (Pm/l)
 |
| | visit 1 | visit 2 | visit 3 |
 urea [mmol/l] urea [mmol/l] | 4.407 | 4.309 | 4.441 |
 urea [mmol/l] urea [mmol/l] | 4.625 | 4.523 | 4.351 |
 urea [mmol/l] urea [mmol/l] | 4.720 | 4.547 | 4.614 |
 creatinine [μmol/l] creatinine [μmol/l] | 65.412 | 66.032 | 66.150 |
 creatinine [μmol/l] creatinine [μmol/l] | 66.918 | 68.100 | 67.393 |
 creatinine [μmol/l] creatinine [μmol/l] | 66.212 | 66.385 | 65.866 |
Diosmin applied in the dose of 2000 mg a day in no way disturbed the basic biochemical parameters of the blood. During the therapy and the next visits, the parameters of urinalysis were assessed, thus confirming no deviations from the correct condition in all the examined groups.
The trial carried out also failed to indicate statistically significant changes in the field of vital signs in the parameter of systolic and diastolic blood pressure for any of the applied doses (tab. 6). Systolic and diastolic blood pressure values measured before the therapy and after 2 months and at the end of the therapy – after 4 months – in all the groups of patients had correct values regardless of the dose applied in the therapy, and small deviations in values were statistically insignificant (tab. 6).
Tab. 6. Blood pressure (mmHg)
 |
| | visit 1 | visit 2 | visit 3 |
 diastolic diastolic | 74.91 | 73.32 | 73.66 |
 diastolic diastolic | 74.94 | 74.50 | 75.40 |
 diastolic diastolic | 74.51 | 74.79 | 73.08 |
 systolic systolic | 118.88 | 118.97 | 121.59 |
 systolic systolic | 118.22 | 118.85 | 121.35 |
 systolic systolic | 118.72 | 119.82 | 118.14 |
The trial proved no occurrence of a serious side effect. Other side effects (most often gastrointestinal issues) were present in all the arms of randomisation, with no statistically significant differences between groups taking the medicine in any dose and placebo, they were episodic and constituted no reason to resign from the therapy.
DISCUSSION
The trial proved that the use of diosmin in doses exceeding the doses commonly recommended in Summaries of Product Characteristics, i.e. doses up to 2000 mg/day for the period of 4 months seems a safe therapeutic option. Larger diosmin doses do not significantly affect complete blood count and biochemical parameters of the blood, the results of urinalysis and blood pressure, which indicates safe for the health and life of patients application of daily doses up to 2000 mg up to 4 months. Not exaggerated and going beyond the safety reasons presented in the introduction is apparently the issue of the above doses being reasonable, though similar theses are also confirmed in the available scientific works and the results of other research confirming the justified therapeutic nature of applying similar doses in various indications and at different times of therapy (11, 17-20).
The results obtained indicate safety of diosmin application in daily dose up to 2000 mg in a therapy lasting up to 4 months. The trial showed no justification for the use of diosmin in the tried indication, however – regardless of the primary goal of the trial, safety of diosmin application was confirmed in the dose up to 2000 mg within 4 months.
CONCLUSIONS
The trial proved no impact of diosmin application in a micronised form in daily dose of 1000 and 2000 mg on the presence of statistically significant differences as regards placebo in the field of any of the examined vital signs for the safety, namely laboratory tests, haematological parameters, biochemical parameters and urinalysis as well as physiological parameters, such as arterial blood pressure.
The results prove the safety of application of a daily dose of diosmin increased to 2000 mg a day and thus they may be utilised to plan further trials using this active substance. The results do not decide about the justified nature of such a dosage, however, revealing such practices in an aware and unaware action of patients or consumers does not have to raise serious concerns as regards safety for the health and life of patients.
Piśmiennictwo
1. Gliński W, Chodynicka B, Roszkiewicz J et al.: The beneficial augmentative effect of micronized purified flavonoid fraction (MPFF) on the healing of leg ulcers: an open, multicentre, controlled, randomised study. Phlebology 1999; 14: 151-157. 2. Howlader M, Coleridge S: Chronic venous disease and venous ulcer: pharmacological approach. J Cardiovasc Dis 2004; 2: 33-41. 3. Shoab SS, Porter JB, Scurr JH, Coleridge-Smith PD: Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg 2000; 31: 456-461. 4. Takase S, Lerond L, Bergan JJ, Schmid-Schönbein GW: The inflammatory reaction during venous hypertension in rats. Microcirculation 2000; 7: 41-52. 5. Ibegbuna V, Nicolaides AN, Sowade O et al.: Venous elasticity after treatment with Daflon 500 mg. Angiology 1997; 48: 45-49. 6. Simka M, Majewski E: The social and economic burden of venous leg ulcers: focus on the role of micronized purified flavonoid fraction adjuvant therapy. Am J Clin Dermatol 2003; 4: 573-578. 7. Barańska-Rybak W, Komorowska O: Zmiany skórne w chorobach naczyń. Forum Medycyny Rodzinnej 2012; 6(1): 35-42. 8. Beckman JA: Diseases of the veins. Circulation 2002; 106: 2170-2172. 9. Chudek J, Ziaja D: Stosowanie preparatów zmikronizowanej diosminy w leczeniu przewlekłej choroby żylnej: raz czy dwa razy na dobę? Chirurgia Polska 2011; 13(2): 132-135. 10. Allegra C, Bartolo M, Carioti B et al.: Microlymphography assessment of Daflon 500 md activity in patients with chronic venous insufficiency. Lymphology 1998; 31 (suppl.): 12-16. 11. Cesarone MR, Laurora G, De Sanctis MT, Belcaro G: Capillary filtration and ankle edema in patients with venous hypertension: effect of Daflon. Angiology 1993; 44: 57-61. 12. Lyseng-Williamson KA, Perry CM: Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs 2003; 63: 71-100. 13. Jain D, Bansal MK, Dalvi R et al.: Protective effect of diosmin against diabetic neuropathy in experimental rats. J Integr Med 2014; 12(1): 35-41. DOI: 10.1016/S2095-4964(14)60001-7. 14. Alvarez N, Vicente V, Martínez C: Synergistic effect of diosmin and interferon-alpha on metastatic pulmonary melanoma. Cancer Biother Radiopharm 2009; 24(3): 347-352. DOI: 10.1089/cbr.2008.0565. 15. Martínez C, Vicente V, Yáñez J et al.: The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol Histopathol 2005; 20(4): 1121-1129. 16. Lewinska A, Siwak J, Rzeszutek I et al.: Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol In Vitro 2015 Apr; 29(3): 417-425. DOI: 10.1016/j.tiv.2014.12.005. 17. Belcaro G, Cesarone MR, Bavera P et al.: HR (Venoruton1000, Paroven, 0-[beta-hydroxyethyl]-rutosides) vs. Daflon 500 in chronic venous disease and microangiopathy: an independent prospective, controlled, randomized trial. J Cardiovasc Pharmacol Ther 2002 Jul; 7(3): 139-145. 18. McHale NG, Hollywood MA: Control of lymphatic pumping: of Daflon 500 m. Phlebology 1994; 9 (suppl. 1): 23-25. 19. Meshikhes AW: Daflon for haemorrhoids: a prospective, multi-centre observational study. Surgeon 2004; 2: 335-338. 20. Roztocil K, Stvrtinová V, Strejcek J: Efficacy of a 6-month treatment with Daflon 500 mg in patients with venous leg ulcers associated with chronic venous insufficiency. Int Angiol 2003 Mar; 22(1): 24-31.

 placebo
placebo 1000 mg
1000 mg 2000 mg
2000 mg
 diastolic
diastolic diastolic
diastolic diastolic
diastolic systolic
systolic systolic
systolic systolic
systolic