*Monika Jabłońska-Jesionowska, Małgorzata Dębska-Rutkowska, Piotr Kwast, Lidia Zawadzka-Głos
Parotitis in children hospitalized in the Department of Pediatric Otolaryngology of the Medical University of Warsaw in the years 2010-2017
Zapalenia ślinianek przyusznych u dzieci w materiale Kliniki Otolaryngologii Dziecięcej WUM w latach 2010-2017
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Wstęp. Wyróżniamy ostre i przewlekłe zapalenia ślinianek przyusznych. Ostre spowodowane są infekcją wirusową lub bakteryjną. Przewlekłe obejmują zapalenia nawrotowe, zapalenia w przebiegu kamicy, zapalenia związane z występowaniem naczyniaków oraz jako objaw chorób autoimmunologicznych.
Cel pracy. Celem pracy była analiza zmian zapalnych występujących u dzieci w obrębie ślinianek przyusznych.
Materiał i metody. W latach 2010-2017 w Klinice Otolaryngologii Dziecięcej Warszawskiego Uniwersytetu Medycznego z powodu obrzęku i bólu ślinianek przyusznych hospitalizowano 30 dzieci. Oceniano wiek, płeć, etiologię zapalenia, lokalizację zmian, wyniki badań dodatkowych oraz współwystępowanie innych chorób.
Wyniki. Przyczyną hospitalizacji był obrzęk i ból ślinianki przyusznej. Wiek dzieci wynosił od 1. miesiąca do 15. r.ż. (średni wiek wynosił 4,2 lata). U 11 dzieci stwierdzono obustronne występowanie zmian, u 19 – jednostronne. U 7 dzieci rozpoznano ostre zapalenie ślinianek przyusznych. Stwierdzono u nich podwyższone wykładniki stanu zapalnego oraz powiększone węzły chłonne lub ropień w obrębie ślinianki w badaniu USG. U 20 dzieci rozpoznano nawrotowe zapalenie ślinianek. U tych dzieci w okresie zaostrzeń nie stwierdzano podwyższonych wykładników stanu zapalnego. W badaniu ultrasonograficznym stwierdzano ogniska hipoechogeniczne. U 11 dzieci rozpoznano choroby o podłożu autoimmunologicznym. U 3 dzieci rozpoznano naczyniaki limfatyczne lub krwionośne. W żadnym przypadku nie stwierdzono kamicy lub pneumoparotitis.
Wnioski. Zapalenie ślinianek przyusznych rozpoczynające się pomiędzy 3. a 4. rokiem życia może być zapaleniem nawrotowym. Rozpoznanie nawrotowego zapalenia ślinianek można postawić na podstawie objawów klinicznych, zmian w badaniu ultrasonograficznym oraz braku podwyższonych wykładników stanu zapalnego. Obustronne, nawracające zapalenie ślinianek przyusznych wiąże się ze zwiększonym ryzykiem występowania chorób o charakterze autoimmunologicznym; zapalenia ślinianek wyprzedzają ich początek. Dzieci z nawrotowym zapalen
Summary
Introduction. Parotitis can be divided into chronic and acute parotitis. Acute parotitis is caused by a viral or bacterial infection. Chronic parotitis includes chronic recurrent parotitis, parotitis due to sialolithiasis, parotitis related to angiomas, as well as parotitis as a symptom of autoimmune diseases.
Aim. The aim of the study was to analyze the inflammatory changes in parotid salivary glands in children.
Material and Methods. Between 2010 and 2017, 30 children were hospitalized in the Department of Pediatric Otolaryngology of the Medical University of Warsaw due to the swelling and pain of a parotid gland . Age, gender, etiology of the disease, location, laboratory findings, and comorbidities were assessed.
Results. The cause of hospitalization was swelling and pain of a parotid salivary gland. The age of children ranged from 1 month to 15 years of age (mean age was 4.2 years). In 11 children, bilateral parotitis was diagnosed, in 19 children, the lesions were unilateral. In 7 children, acute parotitis was diagnosed. In these patients, elevated inflammatory markers, enlarged lymph nodes, or an abscess of the gland in the ultrasonographic examination was observed. In 20 children, chronic recurrent parotitis was diagnosed. In these patients, no elevated inflammatory markers were observed during exacerbations. In ultrasound, hypoechogenic foci were observed. Eleven children were diagnosed with autoimmune diseases. Three children were diagnosed with lymphangioma or hemangioma. None of the patients suffered from sialolithiasis nor pneumoparotitis.
Conclusions. Parotitis that have its onset between 3 and 4 years of age may be recurrent parotitis. The diagnosis of chronic recurrent parotitis is based on clinical symptoms, ultrasound signs, and lack of elevated inflammatory markers. Bilateral chronic recurrent parotitis is related to an incre
Introduction
Parotitis in children is a complex clinical problem. It is characterized by a high number of possible ethiological factors and unpredictable clinical course (1-4). Parotitis can be divided into chronic and acute parotitis.
Acute parotitis in children is usually caused by a viral or bacterial infection. The most common viral etiological factor is mumps virus (2). Other causes include mononucleosis caused by Epstein-Barr virus (EBV), as well as adenoviruses, Coxsackie viruses, influenza virus, and HIV. Bacterial acute parotitis is primarily caused by Staphylococcus aureus and Staphylococcus viridans (2).
Chronic parotitis is less common than the acute one. Chronic parotitis can be divided into the so-called chronic recurrent parotitis, parotitis due to sialolithiasis, and parotitis related to angiomas in the gland. Even less often, parotitis is a manifestation of a systemic or autoimmune disease, such as: sarcoidosis, Sjögren syndrome, cystic fibrosis, diabetes mellitus, and thyroid disorders (1, 2). Moreover, pneumoparotitis can sometimes occur, which can be idiopathic or related to an increased pressure in the oral cavity, most often occurring during balloon blowing, gum chewing, and as a result of professional exposure, for example in trumpeters and glassworkers. In children, pneumoparotitis occurs very rarely (1, 5-7).
Chronic recurrent parotitis in children is a rare disease with a multifactorial and unclear etiology. It is believed that the causes include congenital anomalies of parotid glands and their ducts, as well as recurrent infections, allergies, immunodeficiencies, and autoimmune reactions (2, 8). The role of immunoglobulin IgA is underlined, as its deficiency may lead to chronic parotitis. IgA is an immunoglobulin produced by mucous membranes, including oral and respiratory mucosa. It is thought that chronic recurrent parotitis coexists with respiratory and gastrointestinal diseases, such as asthma and celiac disease (3, 8). Chronic recurrent parotitis is diagnosed in children aged from a few months to 15-17 years of age. The most common symptom is painful swelling of one or two parotid glands without purulent discharge from duct orifice, as well as increased body temperature. The symptoms last for a few days and they resolve spontaneously, reoccurring with various frequency, from a single recurrence to several episodes a year. The diagnosis of chronic recurrent parotitis is often delayed by several months or even years, as the first episode is usually diagnosed as acute parotitis. In parotid ultrasound, characteristic lesions in the form of small hypoechogenic foci are observed, which correspond to parotid duct dilation (3, 4, 9-12). In some cases, chronic recurrent parotitis resolves spontaneously at the onset of puberty – it is then referred to as juvenile recurrent parotitis (2). Treatment method is dependent on the number of recurrences and the severity of clinical symptoms. Between the symptomatic periods, chewing gum, lemon sucking, and other salivary stimulants are advised. It is also recommended to massage and heat the gland, which enables the saliva to liquefy (2, 12). During exacerbations with significant swelling and fever, it may be advisable to introduce antibiotic treatment to prevent adhesions (2, 9, 11-14). In case of a large number of exacerbations and severe symptoms, sialoendoscopy with corticosteroid irrigation of the gland or with sialography, which, in this case, is probably not only diagnostic, but also therapeutic, may be recommended (2, 9, 11-14). The aim of the procedure is to widen parotid ducts or to induce the inflammation of the gland, which result in the exclusion of a part of the gland from saliva production (2, 9, 11-14). Treatment methods also include ligation of the parotid duct and partial superficial parotidectomy. Surgical treatment methods are controversial due to the risks of general anesthesia, the risk of facial nerve paralysis, as well as uncertain clinical outcome with possible recurrence during puberty (2, 9, 12-14).
Aim
The aim of the study was to analyze the inflammatory changes in parotid salivary glands in children, with particular regard to etiology, laboratory findings, and clinical outcome of chronic recurrent parotitis.
Material and methods
Between 2010 and 2017, 30 children were hospitalized in the Department of Pediatric Otolaryngology of the Medical University of Warsaw due to parotitis. For 12 children, the Department of Pediatric Otolaryngology was the first place where they reported to with salivary gland problems. Another 18 children had previously been treated and diagnosed by other departments or their pediatricians. In all the children, sialadenitis was localized in parotid glands. Age, gender, etiology of the disease, location, laboratory findings, and comorbidities were assessed. Laboratory studies included blood count, inflammatory markers (ESR and CRP), serum amylase. Ultrasound examination of the parotid glands was performed. Children with recurrent parotitis had additional tests performed, depending on the number of episodes. The tests included: rheumatoid factor in serum, serum IgA immunoglobulin, and antinuclear antibodies (ANA). In these children, additional endocrine, gastroenterological and rheumatology consultations were planned.
Results
The cause of hospitalization for all 30 children (17 boys and 13 girls) was swelling and pain of a parotid salivary gland. In 12 children (40%), the symptoms were accompanied by elevated body temperature. In 3 children (10%), purulent discharge from duct orifice was observed. Eleven children (36%) were diagnosed with bilateral parotitis, and another 19 (64%) – unilateral parotitis.
In 7 children (5 boys and 2 girls), acute parotitis was diagnosed. In 6 of these patients, inflammation was caused by Staphylococcus aureus, and in 1 patient – by a viral infection in the course of mononucleosis. In this group of patients, 3 children were less than 1 year of age, 1 was 3 years old, and 3 – between 3 to 15 years. In 7 patients, elevated inflammatory markers (leukocytosis, elevated ESR and CRP) were detected. In 5 patients (71%), raised serum amylase was discovered. Six children (86%) had fever. In 3 children (43%), purulent discharge from duct orifice was observed. In ultrasound examination, fine, enlarged lymph nodes were observed, and in 2 children, they were accompanied with an abscess of the gland. First-line treatment consisted of antibiotic therapy. Children with abscesses required additional surgical drainage. Amoxycillin with beta-lactamase inhibitors or cephalosporin with clindamycin were used. In all cases, recovery was achieved and no recurrence was observed.
In 3 children (1 boy aged 6 years and 2 girls aged 3 and 5 months), angioma was suspected after the ultrasound examination. Ultimately, after MRI with contrast, the boy was diagnosed with lymphangioma, and the girls were diagnosed with infantile hemangiomas. The girls were qualified for propranolol treatment and full recovery was achieved. The boy is currently undergoing treatment qualification.
In 20 children (66%; 10 boys and 10 girls aged between 2 and 15 years; mean age 4.4 years), parotitis occurred more than once. In these patients, chronic recurrent parotitis was diagnosed. The age distribution of acute parotitis and the first episode of chronic recurrent parotitis are presented at figure 1.
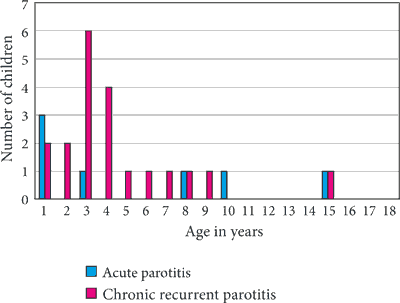
Fig. 1. Age distribution of acute parotitis and the first episode of chronic recurrent parotitis
The frequency of relapses varied: from one recurrence during lifetime to 5-6 times a year. In 9 children (45%), recurrences were always unilateral and usually affected right parotid gland (in 7 children), in another 11 children (55%) the changes were bilateral. In all 20 patients, CRP, ESR and white blood cells count were measured during the acute inflammation. Periodically, a slightly elevated CRP with concurrent normal ESR and normal white blood cells count was observed. Serum amylase in children with chronic recurrent parotitis was within the normal range. In these patients, rheumatoid factor was also measured, and the results were also within the normal limits. IgA level and ANA titer were also assessed. In 2 children with bilateral chronic recurrent parotitis, low IgA level was detected, in 1 patient with unilateral parotitis, high IgA level was identified. In another 3 children, positive ANA results were obtained. In parotid ultrasound, characteristic lesions in the form of small hypoechogenic foci were observed in all 20 children, which corresponds to parotid duct dilation (fig. 2).
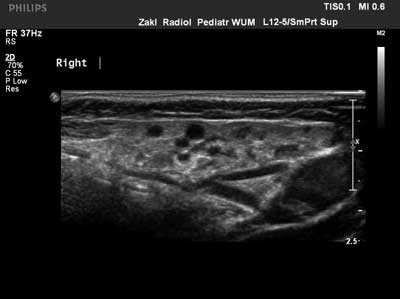
Fig. 2. Chronic recurrent parotitis in ultrasound – hypoechogenic foci
The frequency of recurrences varied: 6 children had 2 episodes, 4 children – 3 episodes, 1 child – 4 episodes, 2 children – 5 episodes, 7 children – several episodes, including 3 children who had several episodes every year. Children with chronic recurrent parotitis periodically report to the Clinic during relapses and stay under constant ENT outpatient care. During the observational period, autoimmune diseases were diagnosed in 12 children (60%) with chronic recurrent parotitis (in 10 patients – 91% – children with bilateral parotitis and in 2 patients – 22% – with unilateral parotitis). Some children have been diagnosed with more than one comorbidity. Parotitis had always preceded the onset of additional symptoms, which were the basis for the diagnosis of other conditions. The comorbidities, along with the frequency of recurrences and localization of parotitis are presented in table 1.
Tab. 1. Autoimmune comorbidities in children with chronic recurrent parotitis and frequency of recurrences and localizations of parotitis; CRP – chronic recurrent parotitis
| Onset of CRP | Number of recurrences | Localization | Comorbidities | Autoimmune diseases in family history |
| 6 years | 2 | bilateral | asthma | - |
| 5 years | 4 | bilateral | allergic rhinitis | - |
| 4 years | 2 | bilateral | asthma
idiopatic arthritis
suspected Sjögren syndrome | - |
| 5 years | 12 | bilateral | Hashimoto’s thyroiditis
diabetes mellitus
celiac disease | - |
| 4 years | 4 | bilateral | allopecia areata | - |
| 4 years | 4 | bilateral | Hashimoto’s thyroiditis | - |
| 8 years | 3 | bilateral | diabetes mellitus | - |
| 10 years | 2 | bilateral | allergic rhinitis
connective tissue disease – under diagnostics | - |
| 16 years | 2 | bilateral | Graves’ disease
lupus-like syndrome
immune thrombocytopenic purpura | - |
| 7 years | 7 (3/year) | bilateral | - | rheumatoid arthritis
Hashimoto’s thyroiditis |
| 3 years | 5 | bilateral | hypothyroidism | - |
| 2 years | 2 | unilateral | systemic sclerosis | - |
| 2 years | 2 | unilateral | autoimmune hemolytic anemia | celiac disease
Hashimoto’s thyroiditis |
The exacerbations of chronic recurrent parotitis that were accompanied with fever and severe, painful swelling were treated with antibiotics. In 9 children who had experienced 5 or more recurrences, sialography of the affected gland was performed as not only a diagnostic method, but also as a possible therapeutic mean.
None of the patients suffered from sialolithiasis nor pneumoparotitis.
Discussion
Parotitis can occur in children of any age. Acute parotitis may occur as early as in the first months of life and does not have a specific peak age. The 7 patients admitted to our Clinic were aged from 1 month to 15 years. Main etiological factor in this group was Staphylococcus aureus bacteria in 6 patients and EBV virus in 1 child, which is confirmed by the literature (1, 2). Clinical signs and symptoms, in addition to painful unilateral or bilateral swelling, included fever of varying degree, as well as elevated inflammatory markers (2). An abscess of the gland frequently occurs, which requires surgical intervention (2). Antibiotic therapy is the treatment method of choice (2). In children with acute parotitis in our study group, no recurrences were observed.
Chronic recurrent parotitis is characterized by a pronounced peak age of ca. 3-4 years of age, when the first episode occurs, which is consistent with our findings (fig. 1). In the literature, the prevalence of this condition is marginally higher in males, which was not confirmed in our analyzed material (10 boys and 10 girls) (2, 9, 11). Despite significant swelling of the parotid gland with accompanying pain and, in rare cases, elevated body temperature, no elevated inflammatory markers are observed (white blood cells count, ESR, and CRP) (2, 9). Similar results were observed in our patients. Diagnostic method of choice is parotid ultrasound, showing characteristic lesions in the form of hypoechogenic foci measuring a few millimeters of diameter (3, 4, 9-12), and these changes occurred in 100% of our patients. The patients periodically require anti-inflammatory treatment. In such cases in our study, antibiotic therapy brought relief as soon as in the 2nd day of treatment. It is thought that antibiotic therapy may prevent recurrence by inhibiting scar formation in the salivary ducts (2, 9-13). Children with bilateral chronic recurrent parotitis, in our material 11 patients, have an increased risk for autoimmune diseases and therefore, require constant endocrine, gastroenterological and rheumatology care. In our material, autoimmune disorders were diagnosed in 10 out of 11 children with bilateral chronic recurrent parotitis (91%). The eleventh child, currently aged 7 years, reported to the Clinic this month. Family history revealed cases of Hashimoto’s thyroiditis and rheumatoid arthritis. The boy is currently undergoing diagnostics according to the outline presented above. In another 10 children, comorbidities such as diabetes mellitus type 1, celiac disease, and Hashimoto’s thyroiditis, as well as isolated cases of alopecia areata and autoimmune anemia, were diagnosed. In 2 children, based on the clinical symptoms accompanying parotitis in the form of xerostomia and eye dryness, Sjögren syndrome was suspected. For this moment, immunochemical tests and histopathological examination had not confirmed the diagnosis in any of the cases. The children are under rheumatology care.
Conclusions
1. Parotitis that have their onset between 3 and 4 years of age may be the first episode of recurrent parotitis.
2. In case of relapses of parotitis, the diagnosis of chronic recurrent parotitis is based on clinical symptoms, characteristic ultrasound sings, and lack of elevated inflammatory markers in spite of swelling, pain and periodically elevated body temperature.
3. Bilateral chronic recurrent parotitis is related to an increased risk of autoimmunological diseases; chronic parotitis precedes their onset.
4. Children with bilateral chronic recurrent parotitis require constant multispecialist care (endocrine, gastroenterological, and rheumatology) and periodic screening for autoimmune diseases.
Piśmiennictwo
1. Baszis K, Tolb D: Reccurent parotitis as a presentation of primary pediatric Sjorgren syndrome. Pediatrics 2012; 129(1): 179-182.
2. Shott SR: Salivary disease in children. In: Cotton RT, Myer ChM (eds.): Practical pediatric otolaryngology. 1st ed., Lippincott-Raven Publishers. Philadelphia 1999: 693-710.
3. Shkalim V, Monselise Y, Mosseri R et al.: Recurrent parotitis in selective IgA deficiency. Pediatr Allergy Immunol 2004; 15(3): 281-283.
4. Leerdam CM, Martin HCO, Isaacs D: Recurrent parotitis of childhood. Journal of J Ped Child Health 2005; 41(12): 631-634.
5. Martín-Granizo R, Herrera M, García-González D et al.: Pneumoparotid in childhood: report of two cases. J Oral Maxillofac Surg 1999; 57(12): 1468-1471.
6. Moënne KB, Cordero JT, Poli CH: Pneumoparotitis or pneumoparotid: a differential diagnosis to consider. Rev Chilena Infectol 2009; 26(6): 555-559.
7. Feure F, Plouin Gaucon I, Tavernier L et al.: A rare presentation of reccurent parotid swelling: self-inducted parotitis. Int J Ped Otol 2009; 4: 29-31.
8. Akar HH, Patıroglu T, Duman L: A selective IgA deficiency in a boy who presented recurrent parotitis. Eur J Microbiol Immunol 2014; 4(2): 144-146.
9. Zenk J, Schneidre H, Koch M et al.: Current management of juvenile recurrent parotitis. Curr Otorhinolaryngol Rep 2014; 2: 64-69.
10. Narsimha RVV, Putta BJH, Kurthukoti AJ: Juvenile recurrent parotitis in children: Diagnosis and treatment using sialography. J Indian Soc Pedod Prev Dent 2014; 32(3): 262-265.
11. Schorr B, Mandel L: Diagnosing juvenile recurrent parotitis. Case Reports. N Y State Dent J 2016; 82(1): 36-39.
12. Capaccio P, Sigismund PE, Luca N et al.: Modern management of juvenile recurrent parotitis. J Laryngol Otol 2012; 126(12): 1254-1260.
13. Nahlieli O, Shacham R, Shlesinger et al.: Juvenile recurrent parotitis: a new method of diagnosis and treatment. Pediatrics 2004; 114(1): 9-12.
14. Roby BB, Mattingly J, Jensen EL et al.: Treatment of juvenile recurrent parotitis of childhood: an analysis of effectiveness. JAMA Otol Head Neck Surg 2015; 141(2): 126-129.

