*Zbigniew Tański, Zbigniew Jarząbek, Bartosz Konowalski, Maciej Truszkowski, Jakub Biedrzycki, Bolesław Kuzaka, Piotr Kuzaka
Fournier gangrene: what’s new in treatment?
Zgorzel Fourniera – co nowego w leczeniu?
Urological Ward, Mazovian Specialistic Hospital named dr Józef Psarski in Ostrołęka
Head of Department: Zbigniew Jarząbek, MD
Streszczenie
Zgorzel Fourniera jest martwiczym, zagrażającym życiu zapaleniem powięzi okolicy krocza i narządów płciowych zewnętrznych oraz odbytu, które może rozciągać się w kierunku jamy brzusznej, prowadząc do martwicy tkanek miękkich i rozwoju sepsy.
Przedstawiono aktualne metody diagnostyki i leczenia płynami, krystaloidami, izotoniczną solą, antybiotykami, sterydami, immunoglobuliną, tlenem hiperbarycznym, opracowaniem chirurgicznym rany i stosowaniem przeszczepów pośredniej grubości martwiczego zapalenia powięzi na podstawie przeglądu piśmiennictwa i wyników leczenia w dwóch Oddziałach Urologii. Początkowo podawano chorym antybiotyki o szerokim spektrum, zgodnie z wytycznymi miejscowych komitetów terapeutycznych oraz z wynikami badań preparatów barwionych metodą Gram i wynikami testów laboratoryjnych. Po otrzymaniu antybiogramu korygowano dalsze leczenie. Wszystkim chorym wytwarzano przetokę nadłonową. Rzadko stosowano opatrunki aktywne.
Zgorzel Fourniera często kończy się zgonem na skutek wystąpienia sepsy, ARDS, niewydolności nerek, wątroby i innych narządów. Nie stosowano leczenia tlenem hiperbarycznym i nie podawano immunoglobulin. Wczesna diagnoza, właściwa chirurgiczna interwencja i zastosowanie odpowiedniego antybiotyku są podstawowe dla zmniejszenia śmiertelności i poprawienia wyników leczenia.
Summary
Fournier gangrene is necrotic, life-threatening fasciitis occurring in the perineal region and within external sexual organs and anus. It may extend to the abdominal cavity, leading to soft tissue necrosis and sepsis.
The article presents current methods of diagnosis and treatment of necrotising fasciitis (Fournier gangrene being a part of this disease) based on the medical literature and experience of two urological wards. The following methods are discussed: isotonic salt and balanced crystalloid fluids, antibiotics, steroids, immunoglobulin, hyperbaric oxygen therapy, surgical debridement and split-thickness mesh grafting.
At the beginning broad spectrum antibiotics were administrated for the patients, according with local guidelines therapeutic committee and results of specimens for Gram’s staining and culture and laboratory tests. After receiving antibiogram prompt antibiotic treatment was continued. Cystostomy was done for everyone. Active dressing was applied rarely. Mortality was not observed in this group of patients.
Fournier gangrene frequently ends with death due to sepsis, ARDS, or insufficiency of the kidneys, liver or other organs. Early diagnosis, careful debridement and application of a proper antibiotic are the basic factors that reduce mortality and improve treatment outcomes.

Introduction
Fournier gangrene is necrotic, life-threatening fasciitis occurring in the perineal region and within external sexual organs and anus. It may extend to the abdominal cavity, leading to soft tissue necrosis and sepsis (1). Type I necrotising fasciitis is caused by a variety of bacteria (2, 3). Type II is caused by one type of bacteria. The disease develops in 0.3-5 cases per 100 thousand people. In research, type II is found in 55-87% of fasciitis cases (4). The microbial spectrum includes anaerobic and aerobic bacteria, Gram-positive and Gram-negative strains, fungi, and even mycobacteria. The most frequently isolated strains are Escherichia coli, Bacteroides, β-haemolytic Streptococcus, Staphylococcus and Proteus. These bacteria settle the gastrointestinal tract, skin and perineal hair follicles. Anaerobic bacteria produce gas in the subcutaneous tissues that causes typical crepitation on palpation. Clostridium infection, which is associated with gas formation, is uncommon, but still has to be taken into account in infections deriving from the colon. Mixed flora has a synergistic action in the development of infection (5).
In developing countries, necrotising fasciitis/Fournier gangrene is very common. Poor economic conditions (malnutrition, famine), wrong treatment of metabolic diseases (diabetes), late treatment initiation, insect and wild animal bites, poor financial capacity, and difficult access to a surgeon result in poor treatment outcomes. For a practicing surgeon (Z.T. – Libya, Nigeria), it was a great challenge to encounter on a daily basis several patients with extensive phlegmon, sepsis and septic shock that required instantaneous intervention (author’s own experience). Some of the patients were diagnosed with AIDS. Mortality in developing countries exceeds 40%. In the European Union, the number of patients with necrotising fasciitis has reduced significantly. In Africa, Fournier necrosis affects men, women and children (author’s experience from hospitals in Adani, Nigeria and Zliten, Libya). In these regions, the incidence is underestimated, particularly in females who are either misdiagnosed or diagnosed late.
However, despite modest hospital resources in these parts of the world, treatment was effective in over 80% of patients. In certain groups of patients (author’s experience from hospitals in Adani, Nigeria and Zliten, Libya), there are no fatal cases.
Pathogenesis of Fournier gangrene
The pathogenesis of Fournier gangrene is characterised by infection with mixed flora, consisting of aerobic and anaerobic bacteria, with subsequent thrombosis and necrosis exacerbated by weakened immunity due to one or several concomitant systemic diseases (2, 4). Aerobic bacteria activate intravascular coagulation cascade, causing aggregation of thrombocytes and complement system, whilst anaerobic bacteria produce heparinases. Thrombosis leads to tissue necrosis and reduces the possibility of local bacterial toxin clearance, thereby intensifying or enhancing reproduction of anaerobic bacteria. Reactive oxygen species (ROS), such as superoxide (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH-), and peroxynitrite (ONOO-), are produced in ischaemic tissues and play a significant role in the pathogenesis. Low ROS concentrations have physiological functions, while higher levels of these substances elicit toxic cell injury, leading to cell destruction. Lower ATP production decreases cellular energy, while DNA damage induces lower protein synthesis. Anaerobic bacteria secrete enzymes and toxins. Lecithinase, kollagenose and hyaluronidase digest fascial surfaces. They produce insoluble hydrogen and nitrogen, thus contributing to gas formation in the subcutaneous tissue, which manifests with crepitation on palpation. Aerobic bacteria generate carbon dioxide, which is soluble and rarely induces subcutaneous gas accumulation. Gram-negative bacterial walls release endotoxins. The activation of macrophages and complement system induces proinflammatory cytokine release and development of septic shock.
Mechanism of necrotising fasciitis
In the 1980s, a relationship was found between the use of non-steroidal anti-inflammatory drugs and group A Streptococcus infection. Non-steroidal anti-inflammatory drugs induce a critical decline of neutrophil function and increase the production of tumour necrosis factor alpha, a key mediator in septic shock (2, 4, 5). Non-steroidal anti-inflammatory drugs mask symptoms of infection and delay the diagnosis and treatment. The source of infection can be determined in 90% of cases. The diagnosis is based mainly on physical examination (6-8). Tissue oedema is seen in 75% of cases, redness in 72%, significant pain in 72%, tissue tension in 68%, fever in 60%, and skin or necrotic bullae in 38% of patients. Fasciitis is distinguished from dermatitis by: recent surgery (although not always), pain that is disproportionate to clinical signs and symptoms, hypotension, skin necrosis, and haemorrhagic bullae in soft tissue infections.
Patients with streptococcal infection without a tangible source. The process starts deep in tissues (2). Worsening pain is the most characteristic, and its occurrence precedes shock and multiple organ failure. Pain may be absent or minor when the patient uses analgesic medications or non-steroidal anti-inflammatory drugs. Patients after surgery or injury may attribute pain to these conditions rather than to acute infections. Pain may be less severe in patients with mental disorders or neuropathy in the course of diabetes. All patients with acute pain, overt or covert route of infection, and fever should be examined for severe soft tissue infection. Local symptoms may be absent or minor (4).
Imaging facilitates diagnosis. Plain radiography helps detect gas. Computed tomography (CT) or magnetic resonance imaging (MRI) present soft tissue oedema in patients with soft tissue infections and demonstrate the presence of gas (9). Crepitation is suggestive of soft tissue infection. Oedema itself may not be an indicator of infection in patients with injury in the examined region or after a recent surgery. It is not therefore a differentiating sign of infection, injury, and inflammation. MRI may show thinning (excessive intensity) of intermuscular fasciae in T2-weighted images. It has been demonstrated to be a sensitive but not specific sign of necrotising fasciitis. In CT performed in patients with documented necrotising fasciitis, the absence of the fascia or the presence of its small fragments are specific for necrotising fasciitis as compared with other muscle and fascia infections (10). Gram-positive staining is paramount for the diagnosis of the cause of infection and for the selection of empirical treatment (11, 12). The material is obtained through a biopsy or, even better, during surgery of necrotic tissue removal. CRP over 200 mg/L (13) and moderate white blood cell left shift with increased creatinine levels and without hypotonia are indicative of alpha Streptococcus infection. Infection can manifest with a leukemoid reaction of 50-150 thousand/mL and sodium level decline below 135 mmol/L. This distinguishes necrotising fasciitis from cellulitis.
A score of the general patient’s state (LRINEC) may speed up the diagnosis of Fournier gangrene (4, 14, 15). It includes white blood cell count, haemoglobin, sodium, glucose, creatinine, and CRP in order to distinguish mild from necrotising fasciitis. LRINEC 5.8 or above in a scale from 0 to 13 with a positive predicting value for necrotising fasciitis reaching 57-92% and negative predictive value of 86-96% in a specificity study for LRINEC is higher in more severe disease (2).
In the urological context, a retrospective study has revealed that procalcitonin may be especially helpful in the diagnosis of urosepsis. The sensitivity and specificity of procalcitonin in urosepsis was 90.3 and 94.3%, respectively, with the cut-off point of 0.3 ng/mL. Despite promising results, their verification in prospective research and in studies involving other urological procedures is necessary (1).
On diagnostic algorithm, early clinical signs and test results are considered in the diagnosis of dermatitis and cellulitis (fig. 1).
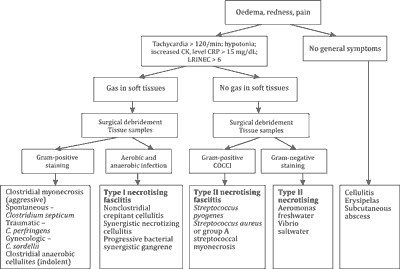
Fig. 1. A diagnostic algorithm for necrotising fasciitis
CK – creatine kinase; CRP – C-reactive protein; LRINEC – Laboratory Risk Indicator for Necrotizing Fasciitis
Treatment
Sepsis: a new consensus from 2016
Sepsis is defined as a life-threatening organ dysfunction caused by abnormal (dysfunctional) systemic reaction to infection.
A simplified definition of sepsis is as follows: sepsis is a life-threatening condition when the reaction to infection damages own tissues and organs.
According to SEPSIS-3, sepsis is associated with organ dysfunction, which indicates much more profound pathophysiological disorders than infections and inflammatory reaction. The current definition of sepsis makes the term “severe sepsis” redundant; the term “sepsis” covers the previous “severe sepsis” (15).
In 1991, the term “systemic inflammatory response syndrome” (SIRS) was introduced. The team developing the new definition conducted studies to assess which examinations are the most appropriate to identify patients with infections that would lead to sepsis (the term “severe sepsis” used to be applied). The criteria included SIRS and two scoring systems that defined organ failure:
– Sepsis-related (Sequential) Organ Failure Assessment Score (SOFA),
– Logistic Organ Dysfunction System (LODS).
In predicting patient ICU mortality, SOFA and LODS scores were more useful than SIRS. This relationship was not observed in patients with suspected infection treated outside the ICU (2, 15).
Septic shock
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Gronostaj K: Postępowanie w urosepsie. Prz Urol 2017; 1(101): 102-111.
2. Stevens DL, Bryan AE: Necrotizing Soft-Tissue Infections. The New Engl J Med 2017; 377: 2253-2265.
3. Wadełek J: Diagnostyka i leczenie zgorzeli Fourniera w oddziale intensywnej terapii. Nowa Med 2016; 23(3): 102-113.
4. Stevens DL: Could nonsteroidal antyflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shok syndrome? Clin Infect Dis 1995; 21: 977-980.
5. McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA: Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 1995; 221: 558-563.
6. Kuzaka B, Borkowski T, Kawecki D et al.: Fournier’s gangrene. Clinical presentation of 13 cases. Mad Sci Monit 2018; 24: 548-555.
7. Kuzaka B, Jardanowski R, Dobroński P: Zgorzel Fourniera. Opis przypadku. Urol Pol 1998; 51(1): 93-100.
8. Siroky MD, Oates RD, Babyan RK: Podręcznik urologii. Diagnostyka i leczenie. Czelej, Lublin 2006: 193-194.
9. Carbonetti F, CremonaA, Carusi V et al.: The role of contrast enhanced computed tomography in the diagnosis necrotizing fasciitis and coparison with Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC). Radiol Med 2016; 121: 106-121.
10. Stamenkovic I, Lew PD: Early recognition of potentially fatal necrotizing fasciitis – the use of frozen-section biopsy. N Engl J Med 1984; 310: 1689-1693.
11. Majeski J, Majeski E: Necrotizing fasciitis: improved survival with early recognition by tissue biopsy and aggressive surgical treatment. South Mad J 1997; 90: 1065-1068.
12. Chelsom J, Halstensen A, Haga T et al.: Necrotising fasciitis due to group A streptococci in western Norway: incidence and clinical features. Lancet 1994; 344: 1111-1115.
13. Wong CH, Khin LW, Heng KS et al.: The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from Rother soft tissue infection. Crit Care Med 2004; 32: 1535-1541.
14. Becha J, Sepehripour S, Hardwicke J et al.: Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score for assessment of early necrotising fasciitis: a systematic review of the literature. Ann R Coll Surg Angl 2017; 99: 341-346.
15. Kübler A: Sepsa – nowa uzgodniona definicja 2016. Chirurgia 2017; 3(133): 19-21.
16. Rhee C, Dantes R, Epstein L et al.: Incidence and trends of sepsis in US hospitals Rusing clinical vs claims data, 2009-2014. JAMA 2017; 318: 1241-1249.
17. Schumer W: Steroids in the treatment of clinical septic shock. Ann Surg 1976; 184: 333-341.
18. Annane D, Bellissant E, Bolleart PE et al.: Corticosteroids for treating sepsis. Cochrane Database Syst Rev 2015; 12: CD002243.
19. Bone RC, Balk RA, Cerra FB et al.: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101: 1644-1655.
20. Venkatesh B, Finfer S, Cohen J et al.: Adjunctive Glucocorticoid Therapy In Patients with Sepstic Shock. The Engl J Med 2018; 378: 797-808.
21. Fadel F, Andrè-Grègoire G, Gravez B et al.: Aldosterone and vascular mineralocorticoid receptors in Marine endotoxic and human septic shock. Crit Care Med 2017; 45(9): e954-e962.
22. Keh D, Trips E, Marx G et al.: Effect of hydrocortisone on development of shock among patients with severe sepsis: the HYPRESS randomized clinical trial. JAMA 2016; 316: 1775-1785.
23. Annane D, Renault A, Brun-Buisson C et al.: Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. The New Engl J Med 2018; 378: 809-818.
24. Self WH, Semler MW, Wanderer JP et al.: Balanced Crystalloids versus Saline In Noncritically Ill Adults. The New Engl J Med 2018; 378: 819-828.
25. Mościcka P, Szewczyk MT, Cwajda-Białasik J et al.: Nowoczesne opatrunki w ambulatoryjnym leczeniu ran zakażonych. Chir po Dypl 2018; 13(3): 36-42.

