Maria Niebrzydowska1, Emilia Duchnowska2, *Paulina Wozniewska3, Piotr Golaszewski3, Regina Sierzantowicz4, Agnieszka Swidnicka-Siergiejko5, Jerzy Robert Ladny1, 3, Jacek Dadan3, Agnieszka Blachnio-Zabielska6, Hady Razak Hady3
Assessment of lipid and carbohydrate balance as well as co-morbidities in patients after adjustable gastric banding in the treatment of obesity
Ocena parametrów gospodarki lipidowej i węglowodanowej oraz chorób towarzyszących u pacjentów poddanych zabiegowi laparoskopowego przewiązania żołądka opaską regulowaną**
1Department of Emergency Medicine, Medical University of Bialystok, Poland
2Department of Clinical Phonoaudiology and Speech Therapy, Medical University of Bialystok, Poland
3Ist Department of General and Endocrine Surgery, University Clinical Hospital, Bialystok, Poland
4Department of Surgical Nursing, Medical University of Bialystok, Poland
5Department of Gastroenterology and Internal Medicine, University Clinical Hospital, Bialystok, Poland
6Department of Hygiene, Epidemiology and Metabolic Disorders, Medical University of Bialystok, Poland
Streszczenie
Wstęp. Najbardziej skutecznym sposobem leczenia otyłości olbrzymiej prowadzącym do poprawy bądź ustąpienia chorób towarzyszących jest chirurgia bariatryczno-metaboliczna. Jedną ze stosowanych metod zabiegowych jest laparoskopowe podwiązanie żołądka opaską regulowaną (LAGB).
Cel pracy. Celem niniejszego badania było przedstawienie wpływu LAGB na BMI, parametry lipidowe, węglowodanowe oraz wątrobowe, a także ocena ustępowania chorób współistniejących u osób poddanych zabiegowi chirurgicznemu.
Materiał i metody. Dokonano oceny 80 otyłych pacjentów (52 kobiety oraz 28 mężczyzn), którzy zostali zakwalifikowani do LAGB. Podczas sześciomiesięcznej obserwacji w określonych odstępach czasowych wykonano pomiary parametrów metabolicznych. Dane zostały przeanalizowane statystycznie.
Wyniki. Średnie wartości BMI uległy zmniejszeniu z 44,12 do 30,36 kg/m2. Osiągnięto także stopniową redukcję stężenia insuliny, glukozy oraz niewielkie obniżenie wartości indeksu HOMA-IR. W przypadku stężenia greliny pierwsze 3 miesiące obserwacji ukazały wzrost jej wartości, a spadek zaobserwowano w 6. miesiącu. Średnie wartości cholesterolu całkowitego, frakcji LDL, HDL oraz trójglicerydów uległy zmniejszeniu. W przypadku parametrów wątrobowych nie uzyskano wyników istotnych statystycznie.
Wnioski. LAGB jest małoinwazyjną procedurą, która pozwala uzyskać znaczny spadek masy ciała, prowadząc do poprawy zdrowia ogólnego pacjentów oraz normalizacji parametrów metabolicznych. W wyniku tych zmian obserwowane jest zmniejszenie objawów zespołu metabolicznego.
Summary
Introduction. The most effective treatment for morbid obesity, leading to improvement or resolution of co-morbidities is bariatric surgery. One of the most widely used method is laparoscopic adjustable gastric banding (LAGB).
Aim. The aim of this study was to present the impact of gastric banding on BMI and serum concentration of ghrelin, insulin, glucose, triacylglycerols, total cholesterol and its HDL and LDL fractions, aspartate and alanine aminotransferase as well as its impact on co-morbidities.
Material and methods. Eighty obese patients (52 women and 28 men) who underwent LAGB have been examined. Metabolic parameters were measured in 6 months follow-up. The results were statistically analyzed.
Results. Reduction in the average BMI from 44.12 to 30.36 kg/m2 after 6 months has been obtained. Gradual decrease of the levels of insulin and glucose has been obtained. Slight reduction in the average value of the HOMA-IR index has been achieved. In the case of ghrelin, the first 3 months of observation brought increase in its value, while the decrease was observed after 6 months. Reduction in average values of total cholesterol and its HDL and LDL fractions and triacylglycerols has been achieved. Statistical significance has not been achieved for changes in ALT and AST in postoperative period.
Conclusions. LAGB is minimally invasive bariatric procedure that provides significant weight loss leading to improvement of the overall health and normalization of metabolic parameters. As a consequence, reduction in the symptoms of metabolic syndrome is observed.

INTRODUCTION
Obesity is one of the most serious health problem in the world. It has reached the size of global epidemic and is currently the most rapidly growing problem in medical, epidemiological, social and economic aspects (1). The most important reason for rapid development of obesity epidemic is automatization of a lifestyle developed countries (1-3). Surprisingly, the number of obese people is increasing rapidly despite the growing awareness of health and easier access to healthy food, diets, nutritionists and fitness instructors promoting healthy and active lifestyle (2, 3).
According to WHO, obesity is defined as a condition of excessive fat accumulation in the form of adipose tissue, that adversely affects health (2). The development of obesity occurs as a result of excessive energy consumption in relation to its demand (4).
The most important causes include: environmental factors, genetic predisposition, endocrine disorders and lifestyle. Aforementioned factors may be genetically determined.
Other causes of obesity are connected with physical activity, type of job, exposure to stress, food availability, the influence of culture, traditions and beliefs, the use of stimulants and drugs, and in addition social factors such as low social status (5).
It is assumed that obese patients reveal damage of physiological mechanisms suppressing appetite. The nutrition is regulated by the central nervous system. Satiety and hunger centers are located in the hypothalamus, limbic system, reticular formation, amygdala and cortex. Functional or organic disorders in these centers may cause changes in dietary habits and lead to obesity (2).
The increase of obesity in the world is connected with the problem of co-morbidities (6, 7). The most important include: type 2 diabetes, arterial hypertension, ischemic heart disease, increased risk of stroke, as well as osteoarthritis and sleep apnea syndrome (7). Obesity adversely influence the quality of life. The development of many chronic diseases of the gastrointestinal tract is observed, such as gastro-esophageal reflux and its complications (Barret’s esophagus, esophageal cancer), polyps and colorectal cancer and liver disease (non-alcoholic fatty liver disease (NAFLD), hepatic cirrhosis, hepatocellular carcinoma) (8, 9). Both, in women and men, obesity is strictly connected with a high percentage of deaths caused by tumors of the esophagus, colon, anus, gallbladder, pancreas, kidney. It increases the risk of death due to stomach and prostate cancer in men and the breast (after menopause), uterus, cervix and ovary cancer in women. The relation of obesity with obstetric failure and depression has also been proved (10). Obesity increases risk of death and a more severe course of chronic diseases – asthma, chronic obstructive pulmonary disease, psoriasis, rheumatoid arthritis, impairs healing process, brings more complications and infections (11, 12). The most serious metabolic complication of morbid obesity is metabolic syndrome.
Bariatric-metabolic surgery is currently the most effective treatment of morbid obesity and leads to the amelioration or resolution of co-morbidities that is achieved by glucose, insulin and lipid metabolism improvement (13). Surgical treatment of obesity should be considered in adult patients with a body mass index (BMI) above 40 or 35 kg/m2 with co-morbidities, where surgically induced weight loss would lead to their improvement or total recovery. Available methods of surgical treatment include sleeve gastrectomy, adjustable gastric banding and gastric bypass.
AIM
The aim of the study was to assess the concentration of total cholesterol and its fractions HDL and LDL, triacylglycerols, glucose, insulin and ghrelin in plasma of patients after laparoscopic gastric banding in 6 months follow-up. The impact of LAGB on BMI and Homeostatic Model Assessment of Insulin Resistance Index (HOMA-IR) was analyzed. The observation of amelioration or resolution of coexisting diseases was also performed.
MATERIAL AND METHODS
The study involved 80 patients after gastric banding performed in the 1st Department of General and Endocrinological Surgery Medical University in Bialystok in 2008-2014 due to morbid obesity.
Patients were qualified for the surgery based on BMI ≥ 40 or ≥ 35 kg/m2 with at least one co-morbidity. Every patient underwent a minimum 6 months attempt of conservative treatment, which was ineffective or unsatisfactory. Patients were prepared for the surgery at the Surgical Outpatient Clinic. Basic laboratory tests were performed – complete blood count, electrolyte and coagulation tests, glucose concentration, insulin concentration, alanine and aspartate aminotransferase concentration, lipidogram, including total cholesterol, its HDL and LDL fractions, triacylglycerols. Additional specialist examinations and consultations were conducted in case of co-morbidities. Data regarding diet and eating habits were gathered in preoperative syndrome.
The study group consisted of 28 (35%) men and 52 (65%) women. The average age of patients was 40 for women and 36 for men. The average body weight was 125 ± 12.3 kg, and the average BMI before the surgery was 44.12 ± 3.21 kg/m2 (tab. 1).
Tab. 1. Characteristics of examined group (mean ± standard deviation)
| Criteria | N = 80 |
| Men/women [%] | 28 (35%)/52 (65%) |
| Age men/women [years] | 36/40 |
| Body mass [kg] | 125 ± 12.3 |
| BMI [kg/m2] | 44.12 ± 3.21 |
In the study group, 15 patients (18.75%) have been treated for type 2 diabetes (oral hypoglycemic agents as well as insulin therapy), 25 patients (31.25%) were treated for hypertension and 4 (5%) suffered from sleep apnea syndrome. Other recorded co-morbidities in study group were osteoarthritis, cholelithiasis, reflux disease and varicose veins. Two patients were treated for infertility.
All procedures have been performed using the laparoscopic method. Patients underwent Swedish adjustable gastrin banding (SAGB). The procedures have been performed using the pars flaccida technique, and four times – perigastric technique. After introducing the Veress needle, a 15-18 mm Hg CO2 peritoneal reflux has been produced. The first trocar on the video track (10 mm) has been introduced above the navel, at a distance of approximately 10 cm between the navel and the left rib arch. The second trocar (10 mm), on the retractor, has been inserted in the median line of the body below xiphoid process of the sternum. Another trocar (5 mm), through which the dissector, coagulation or harmonic knife has been inserted, was placed below the left rib arch in the mid-clavicular line. The incision has been extended in the further stage of the operation to introduce a silicone band into the peritoneal cavity and to implant the port into the subcutaneous tissue. The fourth trocar, used to apply a grasper or goldfinger, has been introduced in the mid-clavicular line under the right rib arch. In some cases, trocar (10 mm) has also been used, inserted in the anterior axillary line at the level of the first trocar for Babcock application. After introducing the trocars to the peritoneal cavity, operating table has been lowered, and the patient has been placed in a semi-high position (semi-Fowler). This position ensures relaxation of the abdominal muscles as well as prevents aspiration of the content to the airways.
The procedure begun with a thorough assessment of the abdominal organs. Using the separator, the back wall of the stomach has been reached along with the angle of His, creating a tube through which a silicone ring has been introduced using a goldfinger and it has been closed on the stomach in the subpylorus area, creating a tank with a volume of approximately 25-40 ml. The band has been adjusted to the front wall of the stomach with two stitches. The procedure terminated by combining the ring with a drain with regulatory port located above the left rib arch in the subcutaneous tissue.
Patients were usually hospitalized for 1 day. They were dismissed with recommendation of 2-week liquid and semi-liquid diet, low-calorie diet, as well as prophylactic antibiotic therapy. In the postoperative period, they received low-molecular-weight heparin and proton pump inhibitors. Dietary recommendations were set by the surgical team in consultation with cooperating dietitians.
Patients underwent 1-month follow-up at the Outpatient Clinic. The first adjustment of the band was usually performed 6-8 weeks after the surgery, and the next at intervals of about 2 months, depending on the rate of weight loss or other. After each adjustment of the band, an attempt to ingest water was made.
The concentrations of ghrelin, insulin, glucose, total cholesterol and its HDL and LDL fractions, triacylglycerols, alanine and aspartate aminotransferase have been measured preoperatively, 7 days, 1, 3 and 6 months after the surgery. Blood samples have been collected on a clot and centrifuged at 4000 rpm for 10 minutes. Serum has been frozen to the temperature of liquid nitrogen and stored at -80°C. Measurements of particular parameters have been conducted using routine methods.
RESULTS
Eighty obese patients after LAGB have been examined. The average pre-operative body weight was 125 ± 12.3 kg, and preoperative BMI was 44.12 ± 3.21 kg/m2.
BMI reduction was noticeable 7 days after the operation – 40.29 ± 3.33 kg/m2. In subsequent measurements, 1, 3 and 6 months after the surgery, a decrease in the mean BMI has also been observed and it was successively 38.15 ± 3.49, 34.59 ± 4.12 and 30.36 ± 5.25 kg/m2. Interestingly, with the decrease in the average BMI value, the deviation from the average value increased each time, from 3.33 kg/m2 7 days after the surgery to 5.25 kg/m2 after 6 months (tab. 2, fig. 1).
Tab. 2. Changes in the concentration of ghrelin, insulin, glucose and changes in mean BMI and HOMA-IR values in a 6-month follow-up after surgery (mean ± standard deviation)
| LAGB | Preoperative concentration | 7 days | p | 1 month | p | 3 months | p | 6 months | p |
| BMI (kg/m2) | 44.12 ± 3.21 | 40.29 ± 3.33* | < 0.01 | 38.15 ± 3.49** | < 0.01 | 34.59 ± 4.12** | < 0.01 | 30.36 ± 5.25** | < 0.01 |
| Ghrelin (pg/mL) | 658.95 ± 175.5 | 647.33 ± 197.75 | NS | 848.76 ± 82.65* | < 0.05 | 924.17 ± 168.41* | < 0.05 | 800.90 ± 148.76 | NS |
| Insulin (μU/L) | 20.71 ± 5.77 | 15.05 ± 2.95* | < 0.05 | 11.47 ± 5.7* | < 0.05 | 9.42 ± 2.70** | < 0.01 | 9.5 ± 0.62** | < 0.01 |
| Glucose (mg/dL) | 103.96 ± 29.76 | 93.23 ± 11.12 | NS | 95.35 ± 5.9 | NS | 96.79 ± 5.87 | NS | 93.2 ± 1.8 | NS |
| HOMA-IR | 6.91 ± 1.37 | 3.95 ± 0.4 | NS | 3.6 ± 0.72 | NS | 3.47 ± 0.74 | NS | 3.77 ± 0.88 | NS |
NS – not statistically significant
*p < 0.05; **p < 0.01
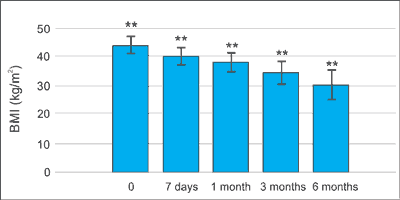
Fig. 1. The course of changes in BMI (mean ± standard deviation)
*p < 0.05; **p < 0.01
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
24 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
59 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
119 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 28 zł
Piśmiennictwo
1. Hady HR, Zbucki R, Luba M et al.: Obesity as a social disease and the influence of environmental factors on BMI in own material. Adv Clin Exp Med 2010; 19(3): 368-378.
2. Nakao YM, Miyamato Y, Ueshima K et al.: Effectiveness of nationwide screening and lifestyle intervention for abdominal obesity and cardiometabolic risks in Japan: The metabolic syndrome and comprehensive lifestyle intervention study on nationwide database in Japan (MetS ACTION-J study). PLoS One 2018; 13(1): e0190862.
3. Regestein QR: The big, bad obesity pandemic. Menopause 2018; 25(2): 129-132.
4. Stokes A, Collins JM, Grant FB et al.: Prevalence and Determinants of Engagement with Obesity Care in the United States. Obesity (Silver Spring) 2018; 26(5): 814-818.
5. Farooqi IS: Defining the neural basis of appetite and obesity: from genes to behaviour. Clin Med 2014; 14(3): 286-289.
6. Dietz WH, Robinson TN: Clinical Practice. Overweight children and adolescents. N Eng J Med 2005; 352(20): 2100-2109.
7. Jakobsen GS Småstuen MC, Sandbu R et al.: Association of Bariatric Surgery vs Medical Obesity Treatment With Long-term Medical Complications and Obesity-Related Comorbidities. JAMA 2018; 319(3): 291-301.
8. Druso JE, Fischbach C: Biophysical Properties of Extracellular Matrix: Linking Obesity and Cancer. Trends Cancer 2018; 4(4): 271-273.
9. Gershuni VM, Ahima RS, Tchou J: Obesity and Breast Cancer: A Complex Relationship. Curr Surg Rep 2016; 4: pii:14.
10. Zarouna S, Wozniak G, Papachristou AI: Mood disorders: A potential link between ghrelin and leptin on human body? World J Exp Med. 2015; 5(2): 103-109.
11. Zewari S, Vos P, van den Elshout F et al.: Obesity in COPD: Revealed and Unrevealed Issues. COPD 2017, 14(6): 663-673.
12. Pharr JR, Coughenour CA, Bungum TJ: An assessment of the relationship of physical activity, obesity, and chronic diseases/conditions between active/obese and sedentary/ normal weight American women in a national sample. Public Health 2018; 156: 117-123.
13. Jastrzebska-Mierzynska M, Ostrowska L, Hady HR at al.: The impact of bariatric surgery on nutritional status of patients. Wideochir Inne Tech Maloinwazyjne 2015; 10(1): 115-124.
14. Hady HR, Dadan J, Gołaszewski P: 100 obese patients after laparoscopic adjustable gastric banding – the influence on BMI, gherlin and insulin concentration, parameters of lipid balance and co-morbidities. Adv Med Sci 2012; 57(1): 58-64.
15. Iskra MP, Ramón JM, Martínez-Serrano A et al.: Unplanned emergency department consultations and readmissions within 30 and 90 days of bariatric surgery. Cir Esp 2018; 96(4): 221-225.
16. Conceiçăo E, Pinto-Bastos A, de Lourdes M et al.: Psychological, behavioral, and weight-related aspects of patients undergoing reoperative bariatric surgery after gastric band: comparison with primary surgery patients. Surg Obes Relat Dis 2018; 14(5): 603-610.
17. Altieri MS, Yang J, Groves D et al.: Sleeve Gastrectomy: the first 3 Years: evaluation of emergency department visits, readmissions, and reoperations for 14,080 patients in New York State. Surg Endosc 2018; 32(3): 1209-1214.
18. Chakravarty PD, McLaughlin E, Whittaker D et al.: Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures: a systematic review of the randomized controlled trials. Surgeon 2012; 10(3): 172-182.
19. Romy S, Donadini A, Giusti V, Suter M: Roux-en-Y Gastric Bypass vs Gastric Banding for Morbid Obesity. Arch Surg 2012; 147(5): 460-466.
20. Gouillat C, Denis A, Badol-Van Straaten P et al.: Prospective, multicenter, 3-year trial of laparoscopic adjustable gastric banding with the MIDBAND. Obes Surg 2012; 22(4): 572-581.
21. Cunneen SA, Brathwaite CE, Joyce C et al.: Clinical outcomes of the REALIZE adjustable gastric bnd-C at one year in a U.S. population. Surg Obes Relat Dis 2012; 8(3): 288-295.
22. Fan J, Xu JH, Wang J et al.: Effects of laparoscopic adjustable gastric banding on weight loss, metabolism, and obesity-related comorbidities: 5-years results in China. Obes Surg 2014; 24(6): 891-896.
23. Saboor Aftab SA, Halder L, Piya MK et al.: Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg 2014; 24(6): 885-890.
24. Carlsson LM, Peltonen M, Ahlin S et al.: Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012; 367(8): 695-704.
25. Praveen Raj P, Bhattacharya S, Saravana Kumar S et al.: Do Bariatric Surgery-Related Type 2 Diabetes Remission Predictors Add Clinical Value? A Study on Asian Indian Obese Diabetics. Obes Surg 2017; 27(8): 2113-2119.
26. Garciacaballero M, Reyes-Ortiz A, Toval JA et al.: Development of type 2 diabetes mellitus thirty-one years after Billiroth II in patient asking for diabetes surgery. Nutr Hosp 2014; 30(1): 219-221.
27. Lee WJ, Hur KY, Lakadawala M et al.: Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg 2012; 16(1): 45-51.
28. Wise ES, Ahmad S, Ladner TR et al.: Impaired weight loss in laparoscopic adjustable gastric banding patients over 50 years of age: diabetes mellitus as an independent risk factor. Surg Endosc 2016; 30(2): 663-669.
29. Sjöström L: Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery. J Intern Med 2013; 273(3): 219-234.
30. Guo X, Liu X, Wang M et al.: The effects of bariatric procedures versus medical therapy for obese patients with type 2 diabetes: meta-analysis of randomized controlled trials. Biomed Res Int 2013; 2013: 410609.
31. Zalewska A, Knas M, Niczyporuk M et al.: Salivary lysosomal exoglycosidases profiles in patients with insulin-dependent and noninsulin-dependent diabetes mellitus. Adv Clin Exp Med 2013; 22(5): 659-666.
32. Ramos-Levi AM, Cabrerizo L, Matia P et al.: Which criteria should be used to define type 2 diabetes remission after bariatric surgery? BMC Surg 2013; 13: 8.
33. Madsbad S, Dirksen C, Holst JJ: Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol 2014; 2(2): 152-164.
34. Bradley D, Conte C, Mittendorfer B et al.: Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest 2012; 122(12): 4667-4674.
35. Adestani A, Rhoads D, Tavakkoli A: Insulin cessation and diabetes remission after bariatric surgery in adults with insulin-treated type 2 diabetes. Diabetes Care 2015; 38(4): 659-664.
36. Gagner M, Deitel M, Kalberger TL et al.: Crosby RD. The Second International Consensus Summit for Sleeve Gastrectomy, March 19-21, 2009. Surg Obes Relat Dis 2009; 5(4): 476-485.
37. Gelisgen R, Zengin K, Kocael A et al.: Effects of laparoscopic gastric band applications on plasma and fundic acylated ghrelin levels in morbidly obese patients. Obes Surg 2012; 22(2): 299-305.
38. Sysko R, Devlin MJ, Schebendach J et al.: Hormonal responses and test meal intake among obese teenagers before and after laparoscopic adjustable gastric banding. Am J Clin Nutr 2013; 98(5): 1151-1161.
39. Wroblewski E, Swidnicka-Siergiejko A, Hady HR et al.: Variation in blood levels of hormones in obese patients following weight reduction induced by endoscopic and surgical bariatric therapies. Cytokine 2016; 77: 56-62.
40. Hallersund P, Sjöström L, Olbers T et al.: Gastric bypass surgery is followed by lowered blood pressure and increased diuresis – long term results from the Swedish Obese Subjects (SOS) study. PLoS One 2012; 7(11): e49696.

