Alicja M. Piotrowska, Magdalena Frąckiewicz, *Lidia Zawadzka-Głos
Assessment of the utility of ultrasonography and microbiology in the treatment of peritonsillar abscess in children
Ocena przydatności ultrasonografii i badań mikrobiologicznych w leczeniu ropni okołomigdałkowych u dzieci
Clinical Department of Paediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Wstęp. Ropnie okołomigdałkowe stanowią najczęstszą infekcję tkanek głębokich w obrębie głowy i szyi. Dotyczą zazwyczaj młodych dorosłych i nastolatków. Pojawiają się przeważnie w sezonie infekcyjnym na przełomie listopada i grudnia oraz kwietnia i maja. Szczyt występowania koreluje z okresem największej zachorowalności na paciorkowcowe zapalenie gardła.
Cel pracy. Analiza kliniczna pacjentów z objawami ropnia okołomigdałkowego pod kątem zasadności wykonywania badania obrazowego ultrasonograficznego potwierdzającego jego obecność.
Materiał i metody. Badanie retrospektywne, nierandomizowane obejmowało grupę 20 pacjentów w wieku od 1 do 17 lat, którzy byli hospitalizowani z rozpoznaniem nacieku lub ropnia okołomigdałkowego na Oddziale Otolaryngologii Dziecięcej. U wszystkich osób z grupy badanej wykonano USG szyi z oceną obecności zbiornika płynowego po stronie prezentowanych objawów.
Wyniki. W grupie badanych pacjentów, w 12 przypadkach w USG szyi opisano przestrzeń płynową wskazującą na obecność ropnia. U wszystkich badanych wykonano nakłucie i nacięcie. W 11 przypadkach uzyskano treść ropną.
Wnioski. Badanie ultrasonograficzne jest pomocne w identyfikacji obecności ropnia, a także potrafi różnicować naciek okołomigdałkowy od ropnia okołomigdałkowego.
Summary
Introduction. Peritonsillar abscess is the most common deep neck and head space infection. It mostly occurs in young adults and teenagers, usually during the infectious season; at the turn of November and December as well as April and May. The peak incidence coincides with the highest incidence of streptococcal pharyngitis.
Aim. A clinical analysis was performed in patients with peritonsillar abscess symptoms to determine the role of ultrasound imaging as a diagnostic tool confirming this condition.
Material and methods. A retrospective, non-randomised study conducted in a group of 20 patients aged between 1 and 17 years, who were hospitalised due to peritonsillar abscess or infiltration in the Department of Paediatric Otolaryngology. Ultrasonography of the neck was performed in all patients in the study group to assess the presence of a fluid reservoir on the symptomatic side.
Results. Ultrasound imaging revealed the presence of fluid indicative of abscess in 12 cases. Incision and puncture were performed in all these patients. Purulent content was obtained in 11 cases.
Conclusions. Ultrasound was found useful in detecting the presence of an abscess and differentiating between peritonsillar infiltration and abscess.
Introduction
Peritonsillar abscess (PTA) is a local complication of acute bacterial tonsillitis. Abscesses develop as a result of infection with anaerobic, aerobic or mixed flora. Group A B-haemolytic streptococci are the most common aetiology (1, 2). The lesion is limited to a single tonsil in most cases (3). Abscess formation follows acute antibiotic-treated or untreated tonsillopharyngitis.
The palatal tonsils, the adenoid, the lingual tonsil, and the tubal tonsils from the Waldeyer’s ring (4).
They are located in a depression between the palatolingual (anterior) and palatopharyngeal (posterior) arches. They are covered by a capsule made of pharyngeal aponeurosis (the superior pharyngeal constrictor muscle) (5). The tonsils are innervated by the glossopharyngeal nerve. They are well-vascularised structures with branches coming from the external carotid artery: the lingual artery, the facial artery with the ascending palatal artery, the ascending pharyngeal artery, and the maxillary artery (4).
The most common symptoms associated with PTA include acute unilateral pharyngeal pain, increasing trismus, gradual muffling of the voice, difficulty in food and drink intake, lack of appetite, and foetor ex ore. Systemic symptoms include deterioration of the general condition and high body temperature. Laboratory findings show increased laboratory markers ? CRP, ESR, procalcitonin and leukocytosis. Laryngological examination usually reveals redness of the throat, coated and asymmetrical palatal tonsils, palatal arch displacement to the midline, and ipsilateral lymphadenopathy (6, 7).
The abscess is usually located in the anterosuperior pole (8). This is accompanied by palatal bulging within the palatolingual arch and inferior-posterior displacement of the tonsil. Other locations include the posterior-inferior arch and the posterior-pharyngeal arch. The treatment of peritonsillar abscess involves surgical intervention.
Aim
We performed a clinical analysis of patients with the symptoms of peritonsillar abscess to determine the role of ultrasound imaging in the diagnosis of PTA (fig. 1).

Fig. 1. An ultrasound scan ? fluid collection in the palatal tonsil
Material and methods
A retrospective non-randomised study was conducted. A total of 20 patients, including 11 females (55%) and 9 males (45%) aged between 1 and 17 years (mean age: 8.9 years), participated in the study. The patients were staying in the Clinical Department of Paediatric Otolaryn-gology of the Medical University of Warsaw between January 1, 2018 and June 30, 2019 due to peritonsillar infiltration or abscess. Neck ultrasound was performed in all patients to verify the presence of fluid reservoir on the symptomatic side.
Results
Ultrasound imaging revealed the presence of fluid indicative of abscess in 12 cases (fig. 2). Incision and puncture were performed in all patients. Purulent content was obtained in 11 cases.

Fig. 2. An ultrasound scan ? an abscess of the palatal tonsil
Ultrasound imaging showed no fluid collection in 8 cases. Puncture of the symptomatic area was also performed in this group, revealing purulent content in one patient despite the lack of ultrasonographic evidence. The patient underwent preoperative neck CT, which showed parapharyngeal abscess (fig. 3).

Fig. 3. Contrast-enhanced neck CT showing an intratonsillar abscess
Surgical intervention (puncture followed by incision and drainage in the case of purulent content) under general anaesthesia was performed in 8 cases. Local anaesthesia was used in the remaining 7 patients. Purulent abscess content was aspirated in a surgical setting in all cases.
The results of microbiological evaluation of the obtained material were analysed. Pathogens were cultured in 83.33% of cases. Physiological flora of the upper respiratory tract was obtained in the remaining patients. Streptococci dominated, with group A Streptococcus pyogenes accounting for 42%. Anaerobic microbes from the group of Prevotella (mainly Prevotella melaninogenica, Prevotella oris and Prevotella bivia) accounted for 25%. Isolated strains accounted for 17%. Infection with Prevotella bivia and methicillin susceptible Staphylococcus aureus (MSSA) was identified in one patient. Other samples contained isolated Staphylococcus aureus with a small percentage of MSSA strains, 8% of Staphylococcus haemolyticus and 8% of Staphylococcus warneri. Physio-logical flora of the upper respiratory tract accounted for 17% (fig. 4).
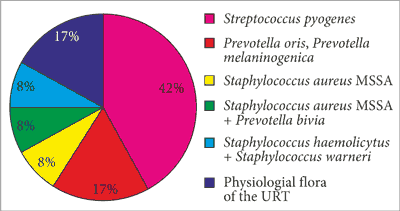
Fig. 4. Percentage involvement of different bacterial strains in the aetiology of peritonsillar abscess in the evaluated patients
Combined IV cefuroxime/clindamycin antibiotic therapy was used for 10 days in 14 cases (70%). Intravenous Augmentin was used in 2 patients. Other patients were put on ceftriaxone and metronidazole or ceftriaxone and clindamycin, and augmentin with clindamycin. Clindamycin alone was used in 1 patient.
Abscesses were unilateral in all patients. About 60% of patients presented with peritonsillar abscess of the right palatine tonsil. Eleven patients were qualified for tonsillectomy, as in accordance with indications (9, 10). Elective tonsillectomy was performed in 7 patients. One patient underwent tonsillectomy during one hospital stay. The other patients are still awaiting surgery.
Histopathological findings confirmed tonsillitis hypertrophica in all cases. The mean age of patients undergoing tonsillectomy was 10.14 years. The youngest patient was 6 years old, and the oldest patent was 16 years old.
Discussion
Peritonsillar abscess is a common reason for reporting to an otolaryngologist. It accounts for the most frequent deep head and neck space infections. The standard management in such cases involves antibiotic therapy and surgical intervention, i.e. abscess puncture and drainage. However, this type of management is problematic in children. Patients of the Department of Paediatric Otolaryngology are not always willing to undergo medical interventions. Fear of the unknown and the symptoms accompanying peritonsillar infiltration do not promote cooperation. Recently, increasingly young patients are reported to hospital due to peritonsillar abscess. The mean age in our study was 10 years. Many of these patients require hospitalisation and surgical treatment under general anaesthesia due to their lack of cooperation during oral antibiotic treatment and abscess puncture. Neck ultrasound is performed to limit unnecessary exposure to stress associated with puncture of the inflamed area. Our analysis showed that ultrasonographic findings correlate with the clinical picture in most cases.
Buckley et al. demonstrated the importance of ultrasound in the differential diagnosis of peritonsillar infiltration vs. abscess. The technique is simple, inexpensive, non-invasive and well-tolerated by patients (11). Bandarkar et al. showed the importance of ultrasonography for identifying peritonsillar abscess, with particular emphasis on children, as well as for qualifying patients for surgical intervention (12).
Our analysis showed that ultrasound is a useful tool for differentiating between tonsillar abscess and peritonsillar infiltration in more than 90% of cases.
Neck ultrasound may be considered useful for making decisions on surgical interventions involving peritonsillar abscess incision.
It should be noted that unilateral asymmetry of the palatine tonsil found during otolaryngological examination may be accompanied by neoplastic process (13). This is the site where primary oropharyngeal tumours are most likely to develop (14). Most palatine tonsil cancers are squamous cell carcinomas and lymphomas. Lymphomas are more common in children (15).
Peritonsillar abscess is often recurrent and is an indication for total tonsillectomy. Patients with tonsillar hypertrophy causing breathing and sleep disorders (total sleep apnea) and recurrent tonsillitis are most often qualified for surgery (4). Generally define as recurrent tonsillitis if there are at least 7 episodes annually or at least 5 episodes in 2 years or there were at least 3 episodes a year in the last 3 years (16). Patients qualified for tonsillectomy belong to one of the above mentioned groups.
Although microbiological findings indicate a multibacterial aetiology of peritonsillar abscess, group A β-hemolytic Streptococcus pyogenes is the most commonly isolated pathogen. This pathogen was isolated from 40% of our patients. However, the world literature points to the growing involvement of anaerobic and mixed flora (1, 2, 17, 18).
All patients received intravenous antibiotic therapy in accordance with treatment standards. ?-lactams in combination with an irreversible β-lactamase inhibitor are the first line treatment (19). However, most patients (70%) were put on cefuroxime/clindamycin combined therapy due to the involvement of anaerobic bacteria, as well as bacterial resistance to the above mentioned antimicrobials.
In addition to peritonsillar abscess, other complications of acute tonsillitis include rheumatic fever and acute glomerulonephritis, which may lead to cardiomyopathy (20). Diagnosed peritonsillar abscess requires intervention in the form of empirical antibiotic therapy followed by targeted antibiotic treatment, as well as surgical intervention, i.e. puncture, incision and drainage. The risk of lack of response is associated with the possible abscess content aspiration after its rupture, airway obstruction and abscess infiltration on the adjacent structures. The proximity to large vessels, such as the internal jugular vein, increases the risk of systemic infection (21). Lemierre’s syndrome, i.e. thrombophlebitis of the internal jugular vein, when the inflamed vessel wall and its compression promotes clot formation, which may cause pulmonary embolism and sepsis, is a very rare one. This syndrome develops secondary to upper respiratory tract infections. Fusobacterium necrophorum, formerly known as Bacillus funduliformis, is the most common aetiological factor (22, 23).
Peritonsillar abscess should be also differentiated against carotid aneurysm, tumour, necrotic lymph node, peritonsillar infiltration and parapharyngeal abscess. A different therapeutic approach is used in such cases.
Ultrasonography is useful in identifying abscess and differentiating peritonsillar infiltration against peritonsillar abscess. Despite the significant role of ultrasound in the dia-gnosis of peritonsillar abscess, medical history and physical examination including, among other things, tonsil palpation, still plays the primary role in the diagnosis.
Conclusions
Ultrasonography is of great diagnostic value in paediatric patients presenting with the symptoms of peritonsillar abscess. The technique is widely available, radiation-free, repeatable and inexpensive. Other imaging modalities, such as head and neck computed tomography, involve exposure of this area to high radiation doses, especially the thyroid, which is highly sensitive to radiation (24).
Piśmiennictwo
1. Brook I, Frazier EH, Thompson DH: Aerobic and anaerobic microbiology of per tonsillar abscess. Laryngoscope 1991; 101(3): 289-292.
2. Brook I: The role of anaerobic bacteria in tonsillitis. Int J Pediatr Otorhinolaryngol 2005; 69: 9-19.
3. Fasano CJ, Chudnofsky C, Vanderbeek P: Bilateral peritonsillar abscesses: not your usual sore throat. J Emerg Med 2005; 29: 45-47.
4. Bohr C, Shermetaro C: Tonsillectomy and Adenoidectomy. Stat Pearls Publishing 2019 Jan 13.
5. Perry ME, Slipka J: Formation of the tonsillar corpuscle. Funct Dev Morphol 1993; 3(3): 165-168.
6. Nguyen T, Haberland CA, Hernandez-Boussard T: Pediatric Patient and Hospital Characteristics Associated With Treatment of Peritonsilla Abscess and Peritonsillar Cellulitis. Clint Pediatr (Phila) 2015; 54(13): 1240-1246.
7. Hsiao HJ, Huang YC, Hsia SH et al.: Clinical features of peritonsillar abscess in children. Pediatr Neonatol 2012; 53(6): 366-370.
8. Janczewski G, Arcimowicz M, Balcerzak J et al.: Powikłania miejscowe i ogólnoustrojowe zapaleń tkanki chłonnej gardła. [W:] Janczewski G (red.): Otolaryngologia praktyczna. Via Medica, Gdańsk 2005: 422-429.
9. Niedzielska G: Postępowanie w nawracających zapaleniach migdałków podniebiennych u dzieci. Otorynolaryngologia 2003; 2(1): 8-10.
10. Burton M: Commentary: Tonsillectomy ? then and now. Int J Epidemiol 2008; 37: 23-25.
11. Buckley AR, Moss EH, Blokmanis A: Diagnosis of peritonsillar abscess: value of intraoral sonography. AJR Am J Roentgenol 1994; 162(4): 961-964.
12. Bandarkar AN, Adeyiga AO, Fordham MT et al.: Tonsil ultrasound: technical approach and spectrum of pediatric peritonsillar infections. Pediatr Radiol 2016; 46: 1059-1067.
13. Jankowska K, Śmiechura-Gańczarczyk M, Konopka W: Jednostronne guzy migdałka podniebiennego ? opis przypadku. Otolaryngologia 2018; 17(1): 36-40.
14. Shah J, Patel S, Singh B (red.): Gardło i przełyk. [W:] Chirurgia i onkologia głowy i szyi. Tom I. Elsevier Urban & Partner, Wrocław 2014: 287-289.
15. Rokkjaer MS, Klug TE: Malignancy in routine tonsillectomy specimens: a systematic literature review. Eur Arch Otorhinolaryngol 2014; 271(11): 2851-2861.
16. Rosenfeld RM, Green RP: Tonsillectomy and adenoidectomy: changing trends. Ann Otol Rhinol Laryngol 1990; 99: 187-191.
17. Zagórski O, Gajda M: Rola beztlenowej flory bakteryjnej w powstawaniu ropni okołomigdałkowych. Pol Merk Lek 2008; XXIV(140): 146-148.
18. Haeggström A, Engquist S, Hallander H: Bacteriology in Peritonsillitis. Acta Oto-Laryngologica 1987; 103(1-2): 151-155.
19. Dzierżanowska D: Przewodnik antybiotykoterapii 2018. Alfa-Medica Press, Bielsko-Biała 2018.
20. Stelter K, Lehnerdt G: Tonsillotomy (partial) and complete tonsillectomy surgical technique. Open Access Atlas of Otolaryngology, Head and Neck Operative Surgery 2017; 1.
21. Bacon E, Tabbut M: When a peritonsillar abscess is not a peritonsillar abscess: using bedside emergency ultrasound to change the diagnosis. Am J Emerg Med 2016; 34(6): 1186.e5-7.
22. Lemierre A: On certain septicemias due to anaerobic organisms. Lancet 1936; 6: 701-703.
23. Eilbert W, Singla N: Lemierre’s syndrome. J Emerg Med 2013; 6(1): 40.
24. Rehrer M, Mantuani D: Identification of peritonsillar abscess by transcutaneous cervical ultrasound. Am J Emerg Med 2013; 31: 267.e1-267.e3.



