Alicja Piotrowska, Magdalena FrД…ckiewicz, *Lidia Zawadzka-GЕӮos
Intracranial complication of acute paranasal sinusitis during active COVID-19 infection – a case report
PowikЕӮanie wewnД…trzczaszkowe ostrego zapalenia zatok przynosowych w przebiegu COVID-19 – opis przypadku
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzk-GЕӮos, MD, PhD
Streszczenie
PowikЕӮania zapaleЕ„ zatok wystДҷpujД… wedЕӮug rГіЕјnych ЕәrГіdeЕӮ z czДҷstotliwoЕӣciД… miДҷdzy 3 a 17%. Do powikЕӮaЕ„ wewnД…trzczaszkowych zaliczamy: zapalenie opon mГіzgowo-rdzeniowych, ropieЕ„ nadtwardГіwkowy, ropieЕ„ podtwardГіwkowy, ropieЕ„ mГіzgu oraz zakrzepowe zapalenie zatok Ејylnych opony twardej i mГіzgu. Do powikЕӮaЕ„ wewnД…trzczaszkowych najczДҷЕӣciej dochodzi w przebiegu zapaleЕ„ zatok czoЕӮowych, sitowych i klinowych. RopieЕ„ mГіzgu jest drugim, po zapaleniu opon mГіzgowo-rdzeniowych, najczДҷstszym powikЕӮaniem wewnД…trzczaszkowym w przebiegu zapalenia zatok przynosowych. Prezentowany przypadek dotyczy 16-letniego pacjenta leczonego z powodu ropni mГіzgu i zakrzepicy zatok Ејylnych OUN w przebiegu ostrego ropnego zapalenia zatoki czoЕӮowej i szczДҷkowej po stronie prawej. PostДҷpowanie w przypadku powikЕӮaЕ„ wewnД…trzczaszkowych ostrego zapalenia zatok przynosowych wedЕӮug EPOS 2022 to wysokie dawki antybiotykoterapii doЕјylnej stosowane przez dЕӮuЕјszy okres, leczenie operacyjne neurochirurgiczne polegajД…ce na drenaЕјu ropni z dostДҷpu przez kraniotomiДҷ, drenaЕјu zatoki czoЕӮowej z dostДҷpu zewnДҷtrznego lub endoskopowego. Rokowanie w przypadku powikЕӮaЕ„ wewnД…trzczaszkowych najczДҷЕӣciej zaleЕјy od nasilenia objawГіw neurologicznych i odpowiedniego szybkiego wdroЕјenia leczenia.
Summary
According to various sources, complications of sinusitis occur with a frequency of between 3 and 17%. Intracranial complications include: meningitis, epidural abscess, subdural abscess, brain abscess, and thrombophlebitis of the dura mater and the brain. The most common intracranial complications are inflammations of the frontal, ethmoid and sphenoid sinuses. A brain abscess is the second most common intracranial complication in the course of sinusitis after meningitis. The presented case concerns a 16-year-old patient treated for brain abscesses and CNS sinus thrombosis in the course of acute purulent inflammation of the frontal and maxillary sinus on the right side. The management of intracranial complications of acute paranasal sinusitis according to EPOS 2022 is high doses of intravenous antibiotics used for a long period, neurosurgical surgical treatment consisting in drainage of abscesses through craniotomy, drainage of the frontal sinus from external or endoscopic access. The prognosis of intracranial complications most often depends on the severity of neurological symptoms and appropriate prompt treatment implementation.
Introduction
Complications of sinusitis occur according to various sources with a frequency of between 3 and even 17%. We distinguish between orbital and intracranial complications and osteomyelitis of the skull. Intracranial complications include: meningitis, epidural abscess, subdural abscess, brain abscess, and thrombophlebitis of the dura mater and the brain. The most common intracranial complications are inflammations of the frontal, ethmoid and sphenoid sinuses. The inflammatory process spreads through thrombophlebitis, through congenital bone defects, through inflamed or necrotic bone, and along lymph spaces or nerves. In some cases, we diagnose more than one intracranial complication of sinusitis (1, 2).
A brain abscess is the second most common intracranial complication in the course of sinusitis after meningitis. Rarely occurs in children under 2 years of age, the frequency increases after 10 years of age. The abscesses are most often located in the frontal lobe as a complication of inflammation of the frontal or ethmoid sinuses (3).
The etiology of brain abscesses is often diverse, the most common are Streptococcus, Staphylococcus and Gram-negative microorganisms, such as Enterobacteriaceae, as well as anaerobic strains (4-6).
Symptoms of a brain abscess can be divided into three groups: general symptoms such as fever, apathy, somnolence, cerebral symptoms resulting from increased intracranial pressure, including headaches, vomiting, and focal symptoms caused by pressure or damage to nerve centers and fibers.
The diagnosis of intracranial complications of sinusitis is based on the analysis of the clinical picture and the results of imaging tests. In radiological diagnostics, computed tomography with contrast and magnetic resonance imaging of the brain are used. Laboratory results are nonspecific: leukocyte counts may be normal and positive blood cultures are obtained in only 10% of cases. Cerebrospinal fluid culture is usually sterile, while lumbar puncture is associated with a high risk of brain stem wedge and is not recommended (4). The etiological factor can be determined from the material collected after drainage of the abscess.
Effective treatment should include intravenous antibiotics and surgery to drain the infected paranasal sinus and decompress the brain abscess. In empirical antibiotic therapy, the following are used: metronidazole with benzylpenicillin or third generation cephalosporin (ceftriaxone or cefotaxime). After the microbiological test results are obtained, targeted antibiotics are used. The duration of treatment depends on the patient’s improvement and the results of imaging tests, and it should last on average 4-8 weeks (2, 4).
One of the factors predisposing to the development of complications of sinusitis is the reduction of immunity after a long-term infection.
Sinusitis with a diagnosed intracranial complication in the form of a brain abscess is a risk factor for another complication – thrombosis of the veins and sinuses of the brain. The clinical picture of this disease depends on the location of the clot. Thrombosis most often includes: superior sagittal sinus (72%), transverse and sigmoid sinus (70%), straight sinus (15%), deep brain veins (8%), cavernous sinus (3%), cerebral cortical veins (2%) and cerebellar veins (2%). The clinical picture is dominated by symptoms of increased intracranial pressure, such as headaches, vomiting, and swelling of the optic nerve discs. Imaging tests are used in diagnostics: computed tomography with contrast, magnetic resonance imaging or angio-MR. Treatment of thrombosis includes anticoagulation, symptomatic treatment of increased intracranial pressure and surgical drainage of the infected paranasal sinus (7).
A case report
A 16-year-old patient was admitted to the Department of Pediatric Otolaryngology from the Department of Pediatrics of the regional hospital due to multiple brain abscesses in the course of acute purulent inflammation of the frontal and maxillary sinus on the right side.
In the interview for a month, the current runny nose, initially mucous, purulent for 10 days. Additionally, headaches in the frontal area have been present for a week. Outpatient treatment with amoxicillin and nasal steroid did not provide improvement. Two days before admission to the hospital ward, paresis of the left lower limb appeared. In hospital treatment, the following intravenous substances were used: Ceftriaxone, Metronidazole, Vancomycin, Dexamethasone, Mannitol and Furosemide. A CT scan of the head performed in a regional hospital described shading of the paranasal sinuses on the right side and an abscess formation in the right frontal lobe. After one day of hospitalization, the patient was transferred to the Department of Pediatric Otolaryngology, UCK, Medical University of Warsaw for specialist treatment.
On admission, the logical and verbal contact was correct. On physical examination, general condition was fairly good. Present slight paresis of the left lower limb. The sinus area was painless on palpation, no protrusions and redness in the paranasal sinuses were observed. No evidence of cervical lymphadenopathy. The abnormalities on physical examination revealed significant bruising of the mucous membranes of the nasal cavities and mucopurulent discharge in the nasal cavities.
The clinic continued intravenous antibiotic therapy: Ceftriaxone 2 g every 12 hours, Vancomycin 500 mg every 6 hours, Metronidazole 500 mg every 8 hours and steroid therapy – Dexamethasone, mannitol and furosemide. Nasopharyngeal swab taken for COVID-19. The obtained PCR test result was positive. Isolation applied. Neurosurgical consultation – urgent neurosurgical intervention was abandoned in order to better organize purulent lesions in the brain. In laboratory tests, prolonged INR and decreased factor VII (36.9%). Hematological consultation was performed to prevent surgery. Qualified for surgical treatment: on the 2nd day of hospitalization, FESS was performed with drainage of the right frontal and maxillary sinus in the COVID + procedure. As recommended by hematologists, Novoseven was administered in a dose of 8 mg immediately before the surgery and continued after the surgery for 7 days. After the procedure, the patient’s condition was stable, after removal of the anterior tamponade, sinus irrigation was performed.
Laboratory tests on day 6 of treatment revealed leukocytosis with neutrophilia (tab. 1) and elevated C-reactive protein 1.46 mg/dl with normal < 0.5 (mg/dl).
Tab. 1. Complete blood count – 7th day of treatment
WBC
(4.0-10.00) | RBC
(4.7-6.1) | HGB
(14.0-18.0)
[g/dl] | HCT
(40-54)
[%] | MCV
(79-95)
[fl] | MCH
(26-32)
[pg] | MCHC
(31-35)
[g/dl] | RDW
(11.5-14.0)
[%] | PLT
(150-400) | MPV
(7.4-11.0)
[fl] | LYMPH%
(24.7-56.0)
[%] | NEUT%
(43-65)
[%] | MONO%
(4.4-12.3)
[%] | BASO%
(0-1)
[%] |
| 13.93 | 5.17 | 13.4 | 40.1 | 77.6 | 25.9 | 22.4 | 13.6 | 180 | 10.5 | 10.8 | 79.7 | 8.7 | 0.5 |
On the 11th day of treatment, a CT of the head with contrast was performed: an abscess in the anteroposterior part of the right frontal lobe, size 34 x 26 x 20 mm, surrounded by a swelling zone, along the front part of the crescent brain several collections fluid – a few (at least three) larger ones on the right side, a few smaller lesions on the left side, lesions partially delimited with a distinct capsule/strengthening, thickened meninges; thrombosis of the right transverse and sigmoid sinus, defects in the filling of the analogous sinuses on the left side; massive changes in the right paranasal sinuses (fig. 1, 2). Additionally, due to the reported pain in the lower left limb and the uncertainty of gait, a Doppler ultrasound was performed. Deep vein thrombosis was found on the left side of the lower limbs, including the femoral, popliteal, sagittal and tibial veins (fig. 3a-e).
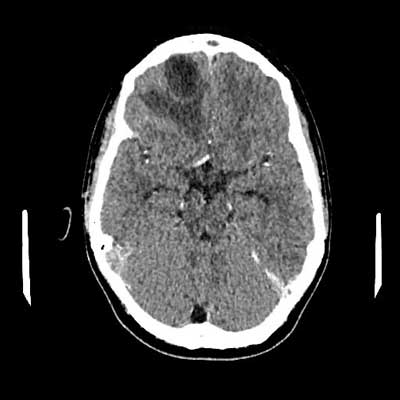
Fig. 1. CT image with contrast showing thrombosis of the sigmoid and transverse sinuses on the right side
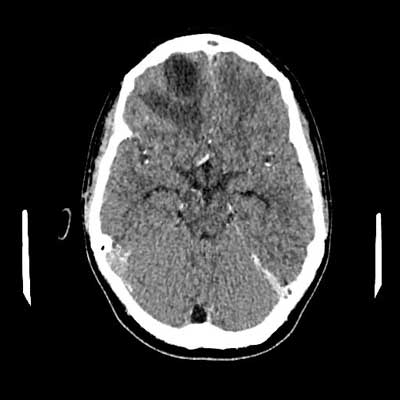
Fig. 2. CT image with contrast
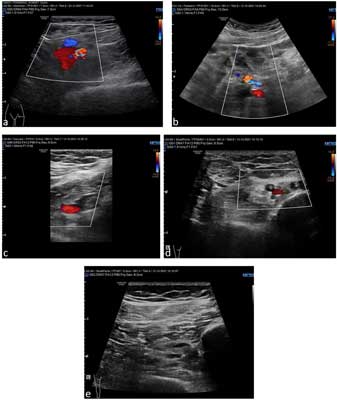
Fig. 3a-e. Doppler ultrasound of the lower extremities – deep vein thrombosis of the lower extremities
Laboratory tests on day 11th and 14th of treatment revealed elevated leukocytosis with neutrophilia according to previous results (tab. 2 and 3).
<Tab. 2. Complete blood count – 11th day of treatment
WBC
(4.0-10.00) | RBC
(4.7-6.1) | HGB
(14.0-18.0)
[g/dl] | HCT
(40-54)
[%] | MCV
(79-95)
[fl] | MCH
(26-32)
[pg] | MCHC
(31-35)
[g/dl] | RDW
(11.5-14.0)
[%] | PLT
(150-400) | MPV
(7.4-11.0)
[fl] | LYMPH%
(24.7-56.0)
[%] | NEUT%
(43-65)
[%] | MONO%
(4.4-12.3)
[%] | BASO%
(0-1)
[%] |
| 19.26 | 5.20 | 13.7 | 40.5 | 77.9 | 26.3 | 33.8 | 14.9 | 135 | 11.6 | 7.4 | 84.5 | 6.7 | 0.1 |
Tab. 3. Complete blood count – 14th day of treatment
WBC
(4.0-10.00) | RBC
(4.7-6.1) | HGB
(14.0-18.0)
[g/dl] | HCT
(40-54)
[%] | MCV
(79-95)
[fl] | MCH
(26-32)
[pg] | MCHC
(31-35)
[g/dl] | RDW
(11.5-14.0)
[%] | PLT
(150-400) | MPV
(7.4-11.0)
[fl] | LYMPH%
(24.7-56.0)
[%] | NEUT%
(43-65)
[%] | MONO%
(4.4-12.3)
[%] | BASO%
(0-1)
[%] |
| 20.32 | 4.26 | 11.4 | 34.0 | 79.8 | 26.8 | 33.5 | 15.5 | 161 | 11.2 | 11.6 | 78.4 | 7.9 | 0.2 |
After haematological consultation, low molecular weight heparin was additionally introduced at a dose of 80 mg every 12 hours. Rehabilitation was applied. In the measurements, the circumference of the thighs: L: 47 cm, P: 48 cm; lower leg: 39.5 cm; P: 39 cm. The measurement on the following day was, respectively, the thigh circumference: L: 48 cm; P: 48 cm; lower leg: 39 cm; P: 39 cm.
On the 18th day of treatment, skin lesions were noticed in the form of a rash on the back and arms, accompanied by itching. Hydrocortison ointment and oral Cetirizine (Allertec) were used locally. Due to the intensification of symptoms during and immediately after the infusion of vancomycin, then ceftriaxone and metronidazole, it was decided to change the intravenous antibiotic therapy to meron.
Qualified for neurosurgery and laryngological surgery, during which the skull trepanation and drainage of CNS abscesses as well as Beck’s puncture of the right frontal sinus were performed. During the procedure 1 unit of Packed cells was transfused without complications. After the procedure, the patient was in a stable general condition. Streptococcus intermedius, sensitive to ceftriaxone, vancomycin and benzyl penicillin, was cultured in the material for microbiological tests. It was decided to maintain monotherapy with meronem antibiotic. During hospitalization, diagnostics for immunity disorders as well as for HIV were performed. The results of the collected tests were normal, HIV negative. In the control Doppler ultrasound, gradual recanalization of changes in the femoral vein was described.
Due to persistent confluent skin eruptions despite anti-histamine therapy and in venosus steroid therapy (Dexaven), after consultation with an infectious disease specialist, decided to change antibiotic therapy to intravenous crystalline penicillin, which was continued for 4 weeks with good results.
During the stay, gradual normalization of inflammatory markers was observed: CRP 0.94 (N: < 0.5 [mg/dl]), results of CBC in the table 4.
Tab. 4. CBC results – 18th day of treatment
WBC
(4.0-10.00) | RBC
(4.7-6.1) | HGB
(14.0-18.0)
[g/dl] | HCT
(40-54)
[%] | MCV
(79-95)
[fl] | MCH
(26-32)
[pg] | MCHC
(31-35)
[g/dl] | RDW
(11.5-14.0)
[%] | PLT
(150-400) | MPV
(7.4-11.0)
[fl] | LYMPH%
(24.7-56.0)
[%] | NEUT%
(43-65)
[%] | MONO%
(4.4-12.3)
[%] | BASO%
(0-1)
[%] |
| 10.10 | 4.28 | 11.4 | 34.6 | 80.8 | 26.6 | 32.9 | 16.8 | 186 | 11.2 | 21.4 | 69.1 | 7.3 | 0.5 |
In the lower limb Doppler ultrasound control, no thrombus was found in the left iliac vein. The system of deep and superficial veins is patent (fig. 4). One may notice restoration of dural sinus patency (fig. 5a-c) and patency of peripheral veins, as well as gradual regression of changes in the attached imaging test results (fig. 6-8). The patient was discharged home with recommended anticoagulant treatment – rivaroxaban.
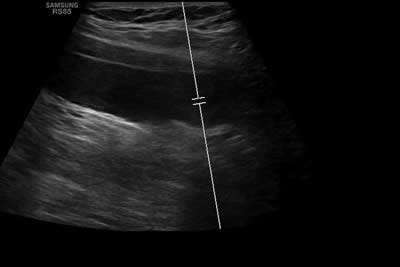
Fig. 4. Doppler ultrasound of lower limbs after treatment
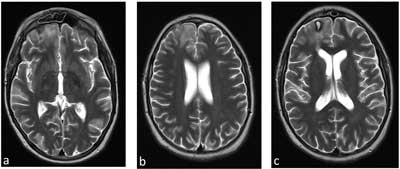
Fig. 5a-c. MRI of the head with contrast after the applied surgical and conservative treatment
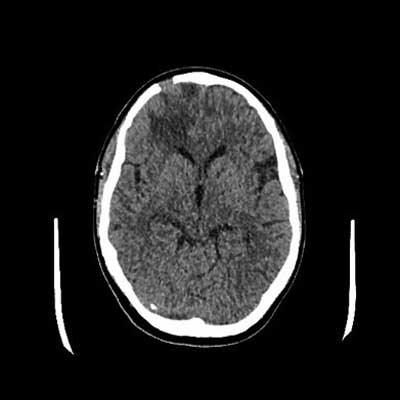
Fig. 6. CT of the head with contrast after treatment, in a follow-up examination
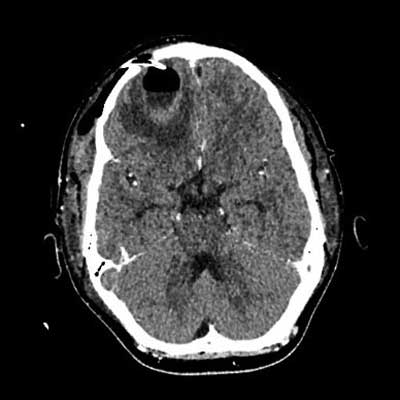
Fig. 7. CT of the head after abscess drainage, condition after craniotomy
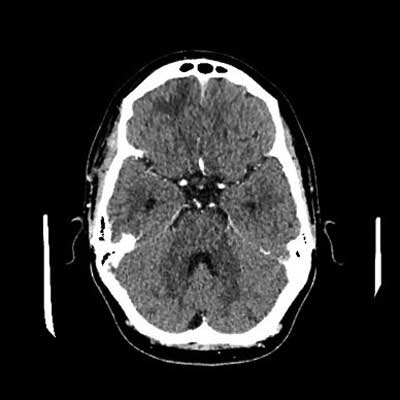
Fig. 8. CT of the head with contrast after treatment
Two months after discharge from the hospital, during the treatment with rivaroxaban – DOAC (direct oral anticoagulants), the patient was urgently admitted to the Department of Hematology of the Children’s Hospital of UCK, Medical University of Warsaw due to bleeding from the oral mucosa and non-specific skin lesions. On admission, the patient was in good general condition, with no active bleeding, no symptoms, no signs of infection. The circumference of the thighs and lower legs are normal. Due to the persistence of thrombus in the venous sinuses of the CNS in imaging studies and the relatively low risk of bleeding in the patient, it was decided to continue rivaroxaban.
The control imaging examinations described the postoperative defects of the frontal and parietal bones on the right side; in comparison to the previous examination, at the level of the defect of the frontal bone in the cortex, a slight hypodense area is slightly more pronounced – it may correspond to the focus of gliosis. Moreover, in the place of the described changes in the right frontal lobe, previously observed hypodense areas of edema smaller than previously, no distinct areas of pathological contrast enhancement can be seen; much smaller, now trace loss of contrast (thrombus) in the transverse and sigmoid sinuses on the right. (fig. 9, 10a, b).

Fig. 9. CT of the head 6 months after the first symptoms
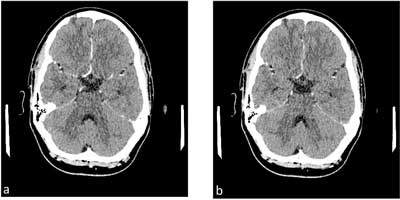
Fig. 10 a, b. Image of sigmoid sinus thrombosis after anticoagulation treatment, examination 6 months from the first symptoms
The patient was in good general condition, with no neurological deficits. He does not report any complaints. It is under constant multi-specialist care.
Discussion
Acute bacterial sinusitis is a common complication of viral upper respiratory infection or severe allergy. According to the EPOS 2020 guidelines, the described case meets the criteria for infection with acute bacterial sinusitis. The symptoms lasted longer than 10 days, the present nasal discharge was mucous. Additionally, they were accompanied by pain in the area of the frontal sinuses, and laboratory tests showed an increased level of CRP (5, 8, 9). Differentiation of bacterial sinus infection from viral infection is possible only after 5 days of infection, when the symptoms worsen, there is a high probability that the inflammation is of a bacterial etiology (10). According to Gou et al. bacterial acute sinusitis is often over recognized (12). Moreover, the early initiation of antibiotic therapy has little or no effect on the development of complications of acute bacterial paranasal sinusitis (ARS) (12). It is estimated that approximately 0.5-2% of viral upper respiratory tract infections are complicated by bacterial inflammation (13, 14).
The alarm symptoms of complications of acute bacterial sinusitis requiring urgent hospitalization include: periorbital edema and erythema, dislocation of the eyeball, diplopia, ophthalmoplegia, decreased visual acuity, edema in the frontal area, symptoms of meningitis, neurological deficits (5). The greatest number of complications occurs between January and March (15). The most common complications concern the orbit in the form of inflammation of the soft tissues in this area, bone complications are much rarer (5, 16).
Intracranial complications of acute paranasal sinusitis are encountered in everyday ENT practice, especially in clinical centers. Although, according to sources, they account for less than 4% of all complications of acute sinusitis. They are more often observed when the inflammatory process is involved in the crush and the frontal sinuses (17). Infection of acute paranasal sinuses can spread from the paranasal sinuses to the intracranial structures in two different ways. Hematological – pathogens can pass through the veins of the skull bone wall to the brain, or spread through the continuity of tissues – pathogens can reach intracranial structures by bone destruction of the thin bony walls of the sinuses (5).
Inflammatory complications of the brain begin with an inflammatory process that leads to progressive necrosis and liquefaction of the brain tissue, which, surrounded by a connective tissue capsule, will form a brain abscess (5). Intracranial complications include subdural empyema, epidural empyema, brain abscess, meningitis, encephalitis, thrombophlebitis of the dura mater (cavernous and sagittal) (5, 18). Brain abscesses can develop from infections originating primarily in the sinuses, teeth, middle ear, and mastoid process. In approximately 20-30% of cases, the source of the infection remains unknown (6).
The most commonly cultured bacteria from the collected material from the cerebrospinal fluid are Streptococcus and Staphylococcus (MRSA) species and anaerobes (5, 6). Among the isolated species, the group of Streptococcus bacteria dominates, most often S. milleria, which includes S. anginosus, S. intermedius and S. constellatus. The isolated Streptococcus intermedius alone accounts for about 50-80% of cases of brain abscess (6, 19). High infectivity and mortality accompanying S. intermedius infection necessitates early antibiotic therapy. In about 90% of cases, this group of bacteria is sensitive to penicillins and in about 98% of cases it is sensitive to second-generation cephalosporins (20).
Management of intracranial complications of acute paranasal sinusitis according to EPOS 2022 is high doses of intravenous antibiotics used for a longer period, surgical neurosurgical treatment consisting in drainage of abscesses through craniotomy, drainage of the frontal sinus from external or endoscopic access (5).
In the presented case, the complications present in the patient were not the most common in acute sinusitis. The complexity of viral infections in the form of the presence of the SARS-CoV-2 virus and the bacterial virus, and the culture of Streptococcus intermedius, cultured from brain abscess material, required complex therapy. The treatment included both i.v. antibiotic therapy, i.v. steroid therapy, anticoagulant therapy and surgery.
The prognosis of intracranial complications most often depends on the severity of neurological symptoms and appropriate prompt treatment implementation (21-23).
Streptococcus intermedius, belonging to streptococci, is often associated with complications of severe upper respiratory tract infections. It can lead to serious complications and are life-threatening, but due to its sensitivity to commonly used antibiotics, it increases the chances of successful treatment (24).
With COVID-19 infection, the possibility of complications such as deep vein thrombosis, pulmonary embolism and bleeding must always be taken possible. It is worth considering the introduction of appropriate antithrombotic prophylaxis. The occurrence of a complication is associated with a higher mortality of patients (25, 26).
Conclusions
Intracranial complications of paranasal sinusitis are still present in ENT practice and this disease should not be forgotten.
In some cases, there may be more than one intracranial complication of sinusitis.
In each case of multiple intracranial complications of paranasal sinusitis, the patient requires a multidisciplinary diagnostic and therapeutic process.
COVID-19 infection is associated with a high risk of thrombotic complications.
PiЕӣmiennictwo
1. GryczyЕ„ska D: Otorynolaryngologia dzieciДҷca. Alfa Medica Press, Bielsko-BiaЕӮa 2007.
2. Janczewski G: Otorynolaryngologia praktyczna. Tom 1. Via Medica, GdaЕ„sk 2007: 335-339.
3. ЕҒebkowski W, Zajkowska J: RopieЕ„ mГіzgu. Neurologia po Dyplomie 2013; 8(5): 38-43.
4. Albrecht P, Hryniewicz W, Kuch A et al.: Rekomendacje postДҷpowania w zakaЕјeniach bakteryjnych oЕӣrodkowego ukЕӮadu nerwowego. Narodowy Instytut LekГіw, Warszawa 2011.
5. Fokkens WJ, Lund VJ, Mullol J et al.: EPOS 2020: European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020; 58 (Suppl 29): 1-484.
6. Mishra AK, Fournier P-E: The role of Streptococcus intermedius in brain abscess. Eur J Clin Microbiol Infect Dis 2013; 32: 477-483.
7. Rajewski P, KsiД…Ејkiewicz B: Zakrzepica ЕјyЕӮ i zatok mГіzgu. Udar MГіzgu 2010; 12 (1-2): 47-50.
8. Wald ER, Applegate KE, Bordley C et al.: Clinical Practice Guideline for the Diagnosis and Management of Acute Bacterial Sinusitis in Children Aged 1 to 18 Years. Pediatrics 2013; 132 (1): e262-e280.
9. Aitken M, Taylor JA: Prevalence of clinical sinusitis in young children followed up by primary care pediatricians. Arch Pediatr Adolesc Med 1998; 152(3): 244-248.
10. Hellings PW, Dobbels F, Denhaerynck K et al.: Explorative study on patient’s perceived knowledge level, expectations, preferences and fear of side effects for treatment for allergic rhinitis. Clin Transl Allergy 2012; 2: 9.
11. Guo M, Alasousi F, Okpaleke C et al.: Prognosis of Chronic Rhinosinusitis With Nasal Polyps Using Preoperative Eosinophil/Basophil Levels and Treatment Compliance. Am J Rhinol Allergy 2018; 32: 440-446.
12. Sleurs K, Seys S, Bousquet J et al.: Mobile health tools for the management of chronic respiratory diseases. Allergy 2019.
13. Pugin B, Deneyer L, Bachert C et al.: Patient Advisory Board for Chronic Rhinosinusitis – A EUFOREA initiative. Rhinology 2019.
14. Hellings PW: Paving the future of rhinosinusitis care. Rhinology 2017; 55: 193-194.
15. Anselmo-Lima WT, Sakano E, Tamashiro E et al.: Rhinosinusitis: evidence and experience: October 18 and 19, 2013 – S.o Paulo. Braz J Otorhinolaryngol 2015; 81 (Suppl 1): S1-S49.
16. Van Dulmen SA, Maas M, Staal JB et al.: Effectiveness of peer assessment for implementing a Dutch physical therapy low back pain guideline: cluster randomized controlled trial. Phys Ther 2014; 94(10): 1396-409.
17. Kakish KS, Mahafza T, Batieha A et al.: Clinical sinusitis in children attending primary care centers. Pediatr Infect Dis J 2000; 19(11): 1071-1074.
18. Khamassi K, Mahfoudhi M, Ben Yahia A et al.: Management of Intracranial Complications of Sinusitis. Open Journal of Clinical Diagnostics 2015; 5: 86-95.
19. Petti CA, Simmon KE, Bender J et al.: Culture-negative intracerebral abscesses in children and adolescents from Streptococcus anginosus group infection: a case series. Clin Infect Dis 2008; 46: 1578-1580.
20. Saijo M, Murono K, Hirano Y, Fujita K: [A patient with Streptococcus intermedius brain abscess treated with high dose penicillin G-susceptibility of the isolate to penicillin G and the concentration of penicillin G in cerebrospinal fluid]. Kansenshogaku Zasshi 1998; 72(4): 414-417. Japanese.
21. Bayonne E, Kania R, Tran P et al.: Intracranial complications of rhinosinusitis. A review, typical imaging data and algorithm of management. Rhinology 2009; 47: 59-65.
22. Clayman GL, Adams GL, Paugh DR, Koopmann CF Jr: Intracranial complications of paranasal sinusitis a combined institutional review. Laryngoscope 1991; 101(3): 234-239.
23. Giannoni CM, Stewart MG, Alford EL: Intracranial complications of sinusitis. Laryngoscope 1997; 107(7): 863-867.
24. Jablonska-Jesionowska M, Zawadzka-Glos L: Children’s head and neck infections caused by Streptococcus intermedius in the Pediatric Otolaryngology Department of Warsaw Medical University. New Medicine 2021; 25(3): 81-86.
25. Katsoularis I, Fonseca-Rodr?guez O, Farrington P et al.: Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: nationwide self-controlled cases series and matched cohort study. BMJ 2022; 377: e069590.
26. Malas MB, Naazie IN, Nadin Elsayed N et al.: Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. Department of Surgery, University of California San Diego Health System, San Diego, CA 92093, United States. E Clinical Medicine 2020; 2930: 10063.









