© Borgis - New Medicine 1/2007, s. 10-14
Ewa Bartkowiak-Fludra1, *Aldona Jasińska-Stępniak1, Marek Gogolewski1, Irena Ponikowska2
Studies on the effect of ozonotherapy on blood serum α-tocopherol level in patients with atherosclerotic ischemia of lower extremities
1Department of Biochemistry and Food Analysis, Agricultural University of Poznań
2Department and Teaching Hospital of Balneology and Metabolic Diseases, University of Medicine, Bydgoszcz
Summary
Summary
The aim of the study was to investigate the destructive effect of ozone on blood serum vitamin E levels in patients with ischemia and macroangiopate dialutica of lower extremities. The primary form in blood serum is α-tocopherol [over 85%], the most biologically active tocochromanol. Sixteen assays were performed in two replications, in which blood serum concentration of vitamin E was determined at the beginning of therapy, after one infusion and after ozonotherapy was completed. Initial vitamin E concentration in the blood serum of patients showed varied vitamin E levels and the occurrence of hypovitaminosis. The action of ozone resulted in individualized vitamin E decomposition, in most cases lowering its level to one below its physiological blood serum content.
Introduction
Vitamin E active compounds, i.e. tocochromanols, are necessary for proper functioning of human and animal organisms. The biological activity of vitamin E is connected with its antioxidant properties, which protect our organisms against harmful action of forming free radicals and fatty acid peroxides [1, 5, 6].
The primary form found in blood serum (over 85%) is α-tocopherol, the most biologically active tocochromanol [moreover, the presence of α- and α-tocopherols was detected].
Alpha-tocopherol embedded into cellular membranes ensures their appropriate structure. It results in proper functioning of the hematopoietic, nervous, reproductive and cardiovascular systems, as well as muscles. It was found that vitamin E is one of the factors inhibiting the process of cell senescence [5, 12, 13].
Blood serum vitamin E concentration below 0.7 mg/dl results in shortening of erythrocyte survival time, neurological disturbances of the immune system and muscular dystrophy. Vitamin E hypovitaminosis increases the likelihood of chronic diseases of the circulatory system, skin and other metabolic disturbances [21]. A positive effect of antioxidants is shown in case of the effect of oxygen free radicals on the development of atherosclerosis. Advantages of the application of vitamin E in the prophylaxis of diseases of the cardiovascular system have been the subject of numerous studies [7]. The risk of disease due to low vitamin E levels might be of bigger importance than classical factors. It was found that low levels of α-tocopherol and ascorbate may result in early angina pectoris. In contrast, vitamin E supplementation in doses above 100 IU/d is connected with statistically significant decrease in the risk of coronary heart disease [15, 17]. Moreover, an advantageous dependency was found between vitamin E intake and the risk of death due to coronary heart disease in post-menopausal women [10].
Vitamin E deficiency may be detected on the basis of increased creatinine concentrations in urine, the erythrocyte hemolysis rate or blood serum α-tocopherol.
One of the factors having a destructive effect on vitamin E is an effect of oxidizing compounds, e.g. ozone. Ozone may oxidize physiologically important compounds and destroy organelles of living cells [3, 4, 11]. It oxidizes e.g. essential unsaturated fatty acids and tocochromanols and other important active reducing substances found in cells [1].
Appropriately selected ozone doses may have an advantageous effect on the functioning of the human organism, which has resulted in its application in the treatment of some diseases in humans and animals [14]. Ozone improves tissue oxygenation and has an advantageous effect on rheological properties of blood [16]. It may also be applied in the treatment of slowly healing wounds and bedsores, in virus and fungal infections, in obliterative thrombosis of lower extremities, in gaseous gangrene and many others, e.g. Arteriosclerosis obliterans, Macroangiopate dialutica [19].
The destructive effect of ozone on vitamin E active compounds, the appropriate blood concentrations of which determine proper functioning of the human organism, constitutes the basis for the concept for this study.
Material
Vitamin E was assayed for diagnostic purposes in patients of the Department and Teaching Hospital of Balneology and Metabolic Diseases in Ciechocinek, of the University of Medicine, Bydgoszcz, diagnosed with the ischemic disease.
A total of 28 patients with symptoms of ischemia of lower extremities due to obliterative atherosclerosis and diabetic macroangiopathy, treated at the above mentioned Department and Teaching Hospital, were included in the study.
Three groups were distinguished out of the patients:
– patients with ischemia of lower extremities – the control group (4 patients not subjected to ozonotherapy);
– patients with ischemia of lower extremities – the experimental group (16 patients undergoing ozonotherapy);
– patients with ischemia of lower extremities – the experimental group (4 patients 10-day vitamin E supplementation in the amount of 200 mg/day and treatment with oxygen-ozone mixture);
– patients with ischemia of lower extremities – the experimental group (4 patients 10-day vitamin E supplementation in the amount of 500 mg/day and treatment with oxygen-ozone mixture).
The patients were 38-75 years old (±60.54 years of age).
The clinical characteristics of the patients, apart from the type of disease, included also the body mass index (BMI), the ankle/shoulder index [A/S index] and total cholesterol (Tables 1 and 2).
Table 1.Clinical characteristics of patients.
| No. | Age | BMI* | Diabetes treatment | A/S Index** before treatment | A/S Index after treatment | Total cholesterol | Type of vascular disease |
| befor | after | R1 | L2 | R1 | L2 | before | after | AO1 | MD2 |
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16 | 53
55
56
47
53
71
75
71
60
61
55
63
69
59
74
67 | 32.0
26.7
32.8
26.2
23.2
22.9
22.3
33.2
20.7
24.0
28.0
24.8
28.0
25.7
34.1
28.5 | 30.0
27.2
30.5
25.2
22.7
22.1
21.9
31.0
20.6
24.0
26.6
24.2
28.0
24.6
32.6
27.8 |
Tablets
Insulin
-
-
-
-
-
Tablets
-
-
-
Insulin
-
Insulin
-
Insulin
| 0.94
-
0.78
0.85
-
-
0.80
1.00
1.03
0.65
0.81
-
0.56
0.53
1.02
0.50 | 0.70
-
0.57
0.76
-
-
0.70
0.90
1.06
0.78
0.66
-
0.50
0.93
1.03
0.80 | 1.06
-
0.84
0.92
-
-
0.80
0.93
1.07
0.69
0.78
-
0.58
0.76
1.00
0.44 | 0.90
-
0.61
0.76
-
-
0.80
0.93
1.06
0.83
0.69
-
0.66
0.96
1.06
0.86 | 214
276
185
307
251
219
238
212
202
248
245
202
192
266
212
189 | 195
289
173
333
253
230
237
186
221
258
218
206
204
276
203
205 |
AO
AO
AO
AO
AO
AO
AO
AO
AO
| MD
MD
MD
MD
MD
MD
MD |
| Control group |
1 control
2 control
3 control
4 control | 52
60
55
74 | 35.1
31.3
22.9
26.6 | 33.50
30.0
22.7
25.8 | Insulin
Tablets
-
Insulin | 0.68
0.94
-
0.36 | 0.72
0.87
0.96
0.94 | 0.71
0.96
-
0.37 | 0.75
0.97
0.93
0.48 | 190
196
295
211 | 171
209
303
208 |
AO
AO | MD
MD
|
BMI* – Body mass index [kg/m2] 25 kg – overweight
AO1 – Arteriosclerosis obliterans
MD2 – Macroangiopate dialutica
A/S.Index** – ankle/shoulder-index; R1 – right, L2 – left
Table 2. Clinical characteristics of patients with vitamin E supplementation in the diet (200 mg and 500 mg/day).
| No. | Age | BMI* | Diabetes treatment | A/S Index** before treatment | A/S Index after treatment | Total cholesterol | Type of vascular disease | Dose/daily intake |
| before | after | R1 | L2 | R1 | L2 | before | after | AO1 | MD2 |
1200
2200
3200
4200 | 38
65
45
62 | 23.0
26.3
25.3
26.6 | 22.5
26.4
25.0
26.2 | -
Insulin
-
- | 0.81
0.92
0.40
0.56 | 0.34
0.60
0.96
0.60 | 1.00
0.94
0.46
0.65 | 0.50
0.64
0.93
0.67 | 262
267
261
243 | 269
233
271
253 | AO
AO
AO |
MD
| 200 mg/day |
5500
6500
7500
8500 | 63
63
60
69 | 37.0
31.6
27.0
15.3 | 36.7
30.2
27.2
15.5 | Insulin
Insulin
Insulin
- | -
0.62
| -
1.06
0.96
- | -
0.85
-
- | -
1.23
1.10
- | 258
206
243
213 | 209
213
250
225 |
AO | MD
MD
MD
| 500 mg/day |
BMI* – Body mass index [kg/m2] 25 kg – overweight
AO1 – Arteriosclerosis obliterans
MD2 – Macroangiopate dialutica
A/S.Index** – ankle/shoulder-index; R1 – right, L2 – left
Methods
The control group consisted of 4 patients (not subjected to ozonotherapy).
Ozonotherapy was administered in case of 16 patients. Assays were performed in three replications, in which blood serum α-tocopherol concentration was determined before the beginning of therapy, after 1 day of therapy and after ozonotherapy was concluded (day 10). The investigations included also 4 patients, which were administered 200 mg vitamin E throughout therapy and 4 patients with diet supplementation of 500 mg vitamin E per day.
The procedure consisted in the dissolution of the oxygen-ozone mixture in isotonic salt solution. The oxygen-ozone mixture contained 95% oxygen and 5% ozone, with ozone concentration of 40-45 μl per 1 cm3 gas. After 10 min ozonization, ozone was dissolved in isotonic salt solution (500 cm3) in the amount of 2000 to 2200 pg. On average the drip infusion lasted for 40-60 minutes. While it was being administered some of the ozone from the solution decomposed and volatilized. In order to maintain the original ozone concentration throughout the administration of the drip infusion the solution was ozonized with the oxygen-ozone mixture.
Blood was collected to test tubes with a clotting agent facilitating prompt separation of blood serum and was subsequently centrifuged for 5 minutes at 3000 rpm.
Vitamin E was assayed using the colorimetric method, based on the Emmerie-Engel reaction. The principle of this method consists in the reduction Fe+3 to Fe+2 by tocopherols, which form a colored complex with 2,2-dipyridyl and the absorbance measurement at wave length of 520 nm. Tocopherols were assayed using a Spekol spectrophotometer equipped with an EK-1 device by Zeiss.
Results and discussion
Ozone in therapy is administered externally in baths in a mixture with oxygen and internally, dissolved in isotonic salt solution or blood and administered intravenously.
The therapeutic action of ozone is connected with the changes induced by the high chemical activity of O3 molecules. Concentrations of compounds susceptible to its action, e.g. vitamin E, change in the organism.
It was proven that ozone administered externally contributes to a decrease in vitamin E concentrations in skin layers [18], whereas in endogenous therapy in patients after myocardial infarction a significant effect of ozone was found on blood lipid metabolism, which contributed to an increase in the activity of antioxidant enzymes [8, 9]. Therapy using the oxygen-ozone mixture in comparison to traditional balneological methods is a more effective method of treating ischemia of lower extremities due to atherosclerosis and diabetes, as evidenced by the improvement in blood supply indexes [20]. Moreover, on the basis of higher malonyldialdehyde concentrations an increased production of oxygen free radicals was found in these patients in comparison to healthy individuals.
In vivo studies on the effect of ozone on changes in human blood α-tocopherol concentrations were also conducted at the Department of Biochemistry and Food Analysis, the Agricultural University of Poznań [2]. Human blood collected from volunteer blood donors was ozonized applying various ozone doses, i.e. 20, 44 and 63 μg/cm3 oxygen-ozone mixture. Moreover, the effect of ozone on blood enriched in vitro with vitamin E was investigated in the study. Statistically significant changes were shown in the concentrations of α-tocopherol both during ozonization (α-tocopherol concentration decreased along with the increase in the ozone dose) and in case of blood samples enriched with vitamin E before ozonization, where the advisability of vitamin E diet supplementation was shown in case of ozone administration for clinical purposes.
In this study the effect of ozonotherapy on blood serum α-tocopherol concentration was investigated in patients with ischemia of lower extremities and the effects of vitamin E oral supplementation during therapy were determined.
In order to determine the repeatability of the conducted investigations 12 simultaneous measurements of carotenoid and vitamin E absorbance were conducted. The error value for carotenoids was 7.0%, while for vitamin E it was 2.1%, respectively. The applied method is burdened with error below ±10%, admissible in this type of determinations.
Blood serum α-tocopherol level in patients not subjected to ozonotherapy was determined three times with 10-day intervals and ranged from 0.52 to 0.94 mg/dl (Table 3).
Table 3. Blood serum α-tocopherol concentration in patients constituting control subjected to 10-day observation.
| Patient No. | a-tocopherol [mg/dl] |
| day 1 | day 10 |
| I | II | III | I | II | III |
| 1 control | 0.84 | 0.76 | 0.92 | 0.79 | 0.83 | 0.93 |
| 2 control | 0.69 | 0.78 | 0.72 | 0.70 | 0.77 | 0.67 |
| 3 control | 0.89 | 0.96 | 0.90 | 0.89 | 0.99 | 0.94 |
| 4 control | 0.47 | 0.56 | 0.56 | 0.49 | 0.57 | 0.52 |
| NS* |
NS* - statistically non-significant
Blood serum α-tocopherol contents in patients with ischemia of lower extremities at the beginning of therapy [before the first drip infusion was administered], after the first and after the tenth drip infusion are presented in Table 4. The concentration of α-tocopherol at the beginning of treatment ranged from 0.32 to 1.00 mg/dl. In six out of sixteen patients (37%) blood serum vitamin E content was lower than that considered necessary physiologically [21]. In two persons its amount (0.31 and 0.34 mg/dl) clearly indicated the incidence of vitamin E deficiency. The occurrence of vitamin E deficiency was found in the 1960´s and it is believed that it was the effect of changes in eating habits, particularly the result of introduction in the diet of bread baked from flour devoid of germ cells containing exceptionally large amounts of α-T homologue, the most biologically active of all tocochromanols. After the administration of the first drip infusion of isotonic salt solution with dissolved ozone, α-tocopherol decomposed in a completely individualized way – from 12 to 65% initial amount, which resulted in the fact that only in 3 patients it remained on the physiologically recommended level.
Table 4. Vitamin E concentration in the blood serum and percent of decomposition after administration of one drip infusion and after completed therapy.
| Vitamin E concentration [mg/dl] |
| Patient No. | Initial | After 1 drip infusion | Vitamin E decomposition [%] | After 10 drip infusions | Vitamin E decomposition [%] |
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16 | 1.00
0.67
0.61
0.57
0.64
0.58
0.34
0.32
0.63
0.57
0.85
0.66
0.78
0.85
0.53
0.95 | 0.66
0.54
0.39
0.20
0.22
0.37
0.23
0.20
0.53
0.50
0.76
0.35
0.52
0.49
0.39
0.64 | 34.00
19.40
36.07
64.91
65.62
36.21
32.35
37.50
18.87
12.28
10.59
46.97
33.34
42.35
26.42
32.63 | 0.58
0.39
0.26
0.10
0.19
0.33
0.20
0.18
0.44
0.38
0.72
0.18
0.38
0.32
0.29
0.43 | 42.00
41.79
57.38
82.46
70.31
43.10
41.20
43.75
30.16
33.33
15.30
72.73
51.28
62.35
45.28
54.74 |
The effect of 10 ozone drip infusions, i.e. the completion of treatment, resulted in a further decrease in serum vitamin E concentrations, in spite of the fact that – as may be assumed – its content in the organism was supplemented from the consumed diet, and also – to a lesser degree – from the amounts stored in the body. Statistically significant differences in α-tocopherol contents were shown between its contents after the first day of therapy in relation to the initial amount and to the amount determined after 10 days, and between α-tocopherol after the completion of therapy in relation to its original level and after the administration of the first drip infusion (Fig. 1).
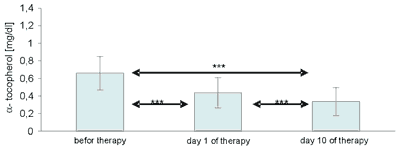
Fig. 1. Statistical differences in blood serum α-tocopherol concentrations in patients receiving ozonotherapy.
In the study blood serum α-tocopherol concentration was investigated in patients receiving vitamin E in the amounts of 200 and 500 mg/day throughout ozonotherapy.
Table 5 lists α-tocopherol values found at the beginning of treatment and after the first drip infusion, as well as after therapy was completed, in patients receiving 200 mg vitamin E per day. The level of α-tocopherol at the beginning of treatment ranged from 0.51 to 0.87 mg/dl. The effect of one drip infusion resulted in the decomposition of α-tocopherol by 25.49 and 33.34%, respectively. As a consequence of the daily supplementation of 200 mg vitamin E its blood serum concentration during therapy decreased by only 6 to 10%, depending on the patient.
Table 5. Vitamin E concentration in the blood serum after daily supplementation of 200 mg and 500 mg of vitamin E and after completed ozone therapy.
| Vitamin E concentration [mg/dl] |
| Patient No. | Initial | After 1 drip infusion | Vitamin E decomposition [%] | After 10 drip infusions | Vitamin E decomposition [%] |
| supplementation of 200 mg |
1200
2200
3200
4200 | 0.64
0.73
0.87
0.51 | 0.50
0.63
0.58
0.38 | 21.87
13.70
33.34
25.49 | 0.58
0.65
0.78
0.48 | 9.37
10.96
10.34
5.88 |
| supplementation of 500 mg |
1500
2500
3500
4500 | 0.68
0.71
0.60
0.84 | 0.30
0.38
0.50
0.58 | 55.88
46.48
16.67
30.95 | 1.12
1.28
1.10
1.25 | 64.70
80.28
83.33
48.81 |
Table 5 also presents blood serum α-tocopherol values for patients receiving 500 mg vitamin E daily. Such a large value of supplemented vitamin E during ozonotherapy resulted in its blood serum level increasing considerably in comparison to its initial level. At the beginning of treatment vitamin E content in the investigated patients ranged from 0.60 to 0.84 mg/dl and corresponded to the recommended values. Blood serum vitamin E levels in patients after one drip infusion dropped by 16.67 and 30.95% respectively. However, after therapy was completed its level for 0.60 was up to 1.1 mg/dl, while for 0.84 - up to 1.25 mg/dl, respectively. It may be concluded that vitamin E supplementation in the amount of 500 mg per day during ozonotherapy made it possible to maintain blood serum α-tocopherol concentration at the level higher than the minimum physiologically recommended requirement.
Statistically significant changes in blood serum α-tocopherol concentrations were observed in three patients, who received 500 mg vitamin E per day, whereas α-tocopherol concentration in patients with the supplementation of 200 mg vitamin E per day did not change in a statistically significant way [Figs. 2].
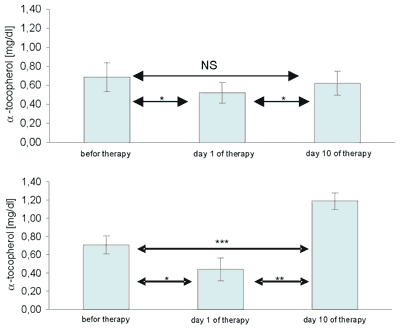
Fig. 2. Statistical differences in blood serum α-tocopherol concentrations in patients receiving ozonotherapy and after 10-day supplementation with 200 mg (a) and 500 mg vitamin E (b).
The studies on the effect of ozonotherapy on blood serum α-tocopherol level there are continuationing and presented results to refer on special group of patients.
Conclusions
1. Initial vitamin E concentration in the blood serum of patients with atherosclerotic ischemia and macroangiopate dialutica of lower extremities showed varied vitamin E levels and the occurrence of hypovitaminosis.
2. The action of ozone resulted in individualized vitamin E decomposition, in most cases lowering its level to one below its physiological blood serum content.
Piśmiennictwo
1. Bartosz G. Druga twarz tlenu. PWN, Warszawa, 1995; 13-295. 2. Bartkowiak-Fludra E. Wpływ wybranych czynników fizyko-chemicznych na tokoferole. Praca doktorska. AR Poznań, 2004. 3. Bocci V. Oxygen - Ozone Therapy: A Critical evaluation. Kluwer Academic Publishers, Medical/Nursing, 2002. 4. Bocci V. Ozone as a bioregulator. Pharmacology and toxicology of ozonetherapy today. J. Biol. Regul Homeost Agents, 1996, 10 [2-3]: 31-53. 5. Combs G.F. Vitamin E, in The Vitamins: Fundamental Aspects in Nutrition and Health. Academic Press Inc., San Diego, 1992. 6. Denisov E.T., Khudykov I.V. Mechanisms of action and reactivities of free radicals of inhibitors. Chem. Revs.,1987; 87: 1313-1357. 7. Gey K., Pusaka P. Vitamin E and vitamin C supplementation. Ann. NY Acad. Sci., 1990; 57: 268-282. 8. Gomez M.F.D., Sazatornil J.A.G., Rosales F.H., Rubi W.D. Effect of a-tocopherol during in vitro ozonation of methyl linoleate: its implication in ozone. Ozone: Science and engineering, 2004; 26: 189-194. 9. Hernandez F., Menendez S., Wong R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endogenous ozone therapy. Free Radical biology and Medicine, 1995; 19, 1: 115-119. 10. Kushi L.H., Folsom A.R., Prineas R.J. Dietery antioxidant vitamins and death from coronary heart disease in posmenopausal women. N. Engl J. Med., 1996; 334: 1152-1163. 11. Liebler D., Matsumoto S., Iitaka Y., Matsuo M. Reaction of vitamin E and ist model compound 2,2,5,7,8-Pentamethylchroman-6-ol with ozone. Chemical Research in Toxicology, 1993; 6: 69-72. 12. Nogala-Kalucka M., Gogolewski M. Związki witamino-E aktywne i ich znaczenie. Polskie Towarzystwo Technologów Żywności, Poznań, 1994; 4. 13. Packer L. Protective Role of Vitamin E in Biological Systems. Am. J. Clin. Nutr., 1991; 53: 1050-1055. 14. Rilling S., Viebahn R. Praxis der Ozon-Sauerstoff-Therapie. Heidelberg, 1990; 48-55. 15. Rimm E.B., Stampfer M.J., Ascherio A. Vitamin E consumption and the risk of coronary disease in men. N Engl. J. Med. , 1993; 328: 1450-1456. 16. Rokitansky O. Der biochemische wirkungs-mechanism der Ozon-Sauerstoff gemisches in blut. Der directe erythrozytare mechanismus. Kongresbericht zur Ozontherapie. Baden-Baden, 1981. 17. Stampfer M.J., Hennekens C.H., Manson J.E. Vitamin E consumption and the risk of coronary disease in women. N Engl. J. Med. , 1993; 328: 1444-1449. 18. Thiele J.J., Traber M.G., Polefka Th.G., Cross C.E., Packer L. Ozone-Exposure Depletes Vitamin E and Induces Lipid Peroxidation in Murine Stratum Corneum. J. Invest Dermatol., 1997; 107: 753-757. 19. Turczyński B. Bezpośredni i odległy wpływ ozonoterapii na lepkość krwi i dystans chromania przystankowego u chorych na cukrzycę. Ann. Acad. Med. Siles., 1992; 25, 105. 20. Włodarczyk K. Badanie enzymów układu antyoksydacyjnego u chorych z przewlekłym niedokrwieniem kończyn dolnych poddanych terapii ozonowej. Praca doktorska. AM Bydgoszcz, 2001. 21. Ziemlański S., Budzyńska-Topolowska J. Tłuszcze pożywienia i lipidy ustrojowe. PWN Warszawa, 1991; 376-388.

