© Borgis - New Medicine 4/2003, s. 117-120
Kornelia Kedziora-Kornatowska1, Hanna Pawluk2, Tomasz Kornatowski3, Leszek Szadujkis-Szadurski3, Robert Pawluk2, Jolanta Czuczejko2, Jadwiga Motyl1, Jozef Kedziora2
Effect of perindopril on plasma carbonyl groups and the level of nitric oxide in elderly patients with essential hypertension
1 Department of Geriatrics, Rydygier Medical University, Bydgoszcz, Poland
Head: Kornelia Kedziora-Kornatowska, MD, PhD
2 Department of Biochemistry, Rydygier Medical University, Bydgoszcz, Poland
Head: Jozef Kedziora, MD, PhD
3 Department of Pharmacology, Rydygier Medical University, Bydgoszcz, Poland
Head: Leszek Szadujkis-Szadurski, MD, PhD
Summary
Introduction: The aim of the study was the measurement of the levels of nitric oxide and carbonyl groups in the plasma of elderly patients with essential hypertension.
Material and methods: Research was done on a control group of 18 healthy patients, and a group of 32 with essential hypertension patients. Prior pharmacological treatment, in case it was used, was stopped 2 days before the research was done. Then all of the patients with primary hypertension were applied perindopril in 4 mg/day dose for the period of 6 weeks. Patients with hypertension were tested after one week and 6 weeks since the start of perindopril therapy. Carbonyls formed by oxidation were determined by the Levine method.
The concentration of proteins in serum was analyzed using the burette method.
The nitrite concentration in plasma was analyzed by the Griess reaction according to Marlett et al.
Results: Hypertensive patients presented a statistically significant (p <0.001) higher carbonyl group concentration both before the therapy (0.25 ± 0.07 nmol/mg protein), and after 7 and 42 days (respectively 0.2 ± 0.08; 0.25 ± 0.07 nmol/mg protein) of perindopril application versus the control group (0.09 ± 0.02 nmol/mg protein). Hypertensive patients showed a lower NO level (1.47 ± 0.63 ?mol/l) compared to the control group (1.91 ± 0.60 ?mol/l), but this difference was not statistically significant.
Conclusions: The results show an increase in oxidative stress, and dysfunction of the vascular endothelium in eldery patients with hypertension
INTRODUCTION
In recent years it has became more common to assume that reactive oxygen species (ROS) participate in both the ageing process and pathogenesis of various diseases of the eldesty age, including essential hypertension (1, 2, 3, 4). ROS have been proved to contribute to damage and dysfunction of vascular endothelium, hypertrophy of cells of vascular walls, and of smooth muscles. On of the main causes of all damage by ROS is the oxidation of both enzymatic and structural proteins. Carbonyl groups resulting from this process are one of the markers of increased oxidative stress (5, 6, 7). Another consequence of ROS activity in primary hypertension can be accelerated reduction of NO (8, 9). Indeed, both in the ageing process and in primary hypertension, vasorelaxation disorders related to endothelium damage have been observed as well as reduced production of NO in the endothelium. A hypothesis has been presented stating that endothelium dysfunction and reduced NO level may result from increased production of a superoxide anion radical. (10). Moreover, it has been observed that in elderly patients with primary hypertension, despite lower plasma renin activity, the activity of the local rennin-angiotensin-aldosteron system including vascular walls, increased. At the same time angiotensin II, playing a key role in the pathogenesis of hypertension, is a factor causing increased generation of ROS. The reaction between the superoxide anion radical and nitric oxide creates peroxynitrite, which is a strong oxidant initiating the peroxidation process (11). Inactive NO from the reaction with ROS and consequent dysfunction of the vascular endothelium can play a role in the pathogenesis of hypertension. The effects of antioxidant use can be observed particularly in elderly patients (12, 13). It is important to note that the activity of the vascular endothelium can be modulated by various pharmacological preparations. There are a few reports on oxidant stress modifying factors in elderly patients with intrinsic hypertension. Some hypertension medicines are reported to have encouraging effects (14, 15). However, it isn´t clear whether the results of these medicines are related to medicine´s hypertension mechanism and/or their modifying influence on certain metabolic processes.
The aim of the study was measurement of selected parameters of the oxygenic metabolism in the blood of elderly patients with arterial hypertension by measurement of the concentration of carbonyl groups in the proteins and concentration of nitric oxide (NO2-, NO3-) before and after treatment.
MATERIAL AND METHODS
The research was conducted on a control group of 18 healthy patients aged 65-96, and a group of 32 patients aged 65-96 with essential hypertension.The clinical characteristics of the patients are shown in Table 1. Research excluded patients declaring high alcohol consumption, addicted tobacco smokers, and those with diabetes, ischaemic heart disease, stroke history, kidney insufficiency, and other conditions of known free radical etiology. Prior pharmacological treatment, in case it was used, was stopped 2 days before the research was done. Then all of the patients with primary hypertension were applied perindopril in 4 mg/day dose for the period of 6 weeks. Patients were under the constant care of doctors in the Geriatric Clinic of the Medical Academy in Bydgoszcz. Hypertensive patients were tested after one week and 6 weeks from the start of perindopril therapy.
Table 1. Characteristics of study subjects.
| Parameters | Group I | Group II |
| Glucose, mg/dl | 101,6 ? 9,8 | 76,4 ? 9,4 |
| Cholesterol | 223,4 ? 39,7 | 212,5 ? 37,9 |
| HDL cholesterol, mg/dl | 58,6 ? 3,4 | 47,8 ? 9,7 |
| Trigliceride, mg/dl | 113 ? 32,8 | 130,9 ? 49,5 |
| Systolic pressure mmHg | 119,3 ? 10,5 | 164,2 ? 15,4* |
| Diastolic pressure mmHg | 71 ? 4,7 | 91,3 ? 6,8* |
| Creatinine, mg/dl | 1,2 ? 0,4 | 1,05 ? 0,7 |
* p <0,05 (group I vs groupII)
Carbonyls formed by oxidation were determined by the Levine method (16).
The concentration of proteins in serum was analysed using the burette method (17). The concentration of nitrites in serum was analysed by the Griess reaction according to Marlett et al (18).
RESULTS
Statistically significant higher systolic and diastolic blood pressures were observed in the group with hypertension in comparison with the normotensive group.
Baseline mean systolic and diastolic blood pressures were 119.3 ± 10.5 and 71.0 ± 4.7 mmHg in the control group, and 164.2 ± 15.4 and 91.3 ± 6.8 mmHg from essential hypertensive patients. Other clinical characteristics were not significantly different either among normotensive subjects or among patients with essential hypertension (Table 1). The concentration of carbonyl groups and nitric oxide (NO2-, NO3-) shown by elderly patients with primary hypertension is presented in Table 2.
Table 2. Results of the hypertensive patients and normotensive controls.
| Perindopril |
| Control | Before treatment | After treatment 7 days | After treatment 42 days |
| protein carbonyls (nmol/mg) | 0,09 ? 0,02 | 0,25 ? 0,07 | 0,26 ? 0,08 | 0,25 ? 0,07 |
| NO (mmol/l) | 1,91 ?0,60 | 1,47 ? 0,63 | 1,36 ? 0,74 | 1,72 ?0,89 |
Hypertensive patients presented statistically significant (p <0.001) higher carbonyl group concentrations both before the therapy (0.25 ± 0.07 nmol/mg protein), and after 7 and 42 days (respectively 0.26 ± 0.08; 0.25 ± 0.07 nmol/mg protein) of perindopril application versus the control group (0.09 ± 0.02 nmol/mg protein – Fig. 1). No statistically significant differences in carbonyl group concentrations between the hypertensive patients before and after perindopril therapy were observed. Hypertensive patients showed a lower NO (NO2-, NO3-) release from vascular endothelium (1.47 ± 0.63 ?mol/l) compared to the control group (1.91 ± 0.60 ?mol/l), but the difference was not statistically significant. After 42 days of perindopril therapy NO concentration showed a growing tendency (1.72 ± 0.89 ?mol/l). Again the difference was not statistically significant when compared to either the control group or to the period before therapy (Fig. 2).
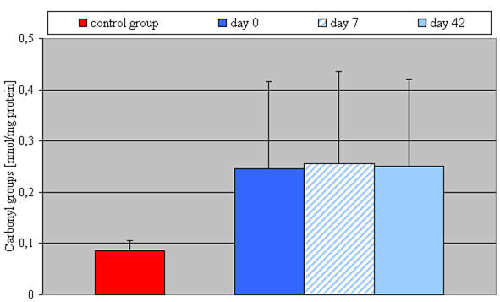
Fig. 1. Plasma concentration of carbonyl groups in control group and in patients with essential arterial hypertension before and after perindopril therapy.
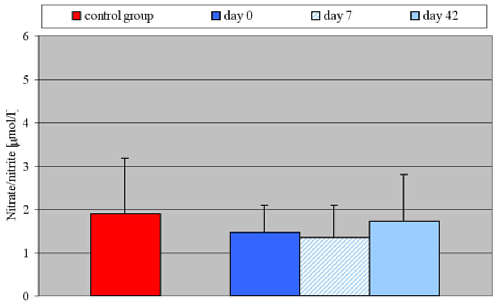
Fig. 2. Serum concentration of nitrate/nitrite in control group and in patients with essential arterial hypertension before and after perindopril therapy.
DISCUSSION
According to the free-radical ageing theory proposed in 1956 by Denham Harman, the ageing process results in increased production of ROS. On the one hand it leads to biological ageing of the cells, but on the other causes a number of medical conditions, among which is intrinsic hypertension (4). Carbonyl groups are the markers for protein damage by ROS. Numerous research projects indicate that their number increases with age (6, 19). Particularly high concentrations are observed in hypertension. This points to the ROS as one of the principal, if not the most important, pathogenic factors leading to intrinsic hypertension. Agarwal and Sorhal (20) also claim that carbonyl groups are one of the key bio-markers in age-related oxidant cell damage and other conditions found in the elderly.
It has also been proven that hypertension is characterised by increased concentrations of other oxidative stress markers. In uncontrolled hypertension the level of superoxide anion radical, hydrogen peroxide, and lipid peroxide are higher than in healthy patients (4).
Many researchers have proved that the factor responsible for stimulating the production of superoxide anion radical is oxydase NADPH. A genetic origin for the expression of the enzyme is also suggested (gen P-22). Humoral factors, such as angiotensin II (AT II) can also be responsible for the changes in the enzyme´s activity. Blocking the AT1 receptor for angiotensin II with losartan practically stops the production of superoxide anion radical after stimulating with oxydase NADPH. Applying AT II in small doses leads to increased vascular pressure through stimulating oxidative stress – measured by an increase in the 8-iso PGF2 alfa level. AT II can also stimulate the production of endothelins which stimulate oxidative stress, which in consequence reduces the NO concentration (21).
In our own research, increased concentration of carbonyl groups in normal tension elderly patients was confirmed. Particularly it was observed in group of elderly with essential hypertension. It indicates an increase of oxidative stress in the ageing process, especially when it coexistences with essential hypertension.
Perindopril therapy has not caused significant differences in the distribution of carbonyl group concentrations in the research groups. However, the antioxidant properties of angiotensin convertase inhibitors are confirmed by our own earlier research as well as research by other authors. It has been shown that angiotensin convertase inhibitors slow down lipid peroxidation processes and ROS generating processes (22, 23). This research did not relate to elderly patients who suffered from overlapping between ageing process-related strong free radical reactions and pro- and antioxidative homeostase dysfunctions related to hypertension.
Our research shows that hypertensive patients experience reduced levels of NO release, as measured by the concentration of final products of NO metabolism in comparison to the control group. Similar results were obtained by Node et al (24), who proved a reduced NO concentration in hypertensive patients. Plasma NO level in patients with hypertension from other age groups has also been reduced compared to control groups. An interesting observation has been made by Kumar and Das, who have shown in their in vitro research that NO could, regardless of a vasodilating effect, indirectly slow down angiotensin convertase activity (25).
Blocking angiotensin convertase with enalapril resulted in increased activity of catalase and GSH-Px. Certain researchers have not confirmed the positive influence of angiotensin converting enzyme inhibitors and antagonists of the AT1 receptor on the endothelium function and concentration of NO. In our research, after 42 days of perindopril therapy we also observed an increased tendency to generate NO. Increased plasma NO concentration after therapeutic use of angiotensin convertase inhibitors with intrinsic hypertensive patients has also been indicated by other authors (15, 26). However, these researches have not been conducted upon elderly patients with hypertension, i.e. those whose dysfunction of the vascular endothelium results from both the ageing process and accompanying hypertension. Therefore, it is possible that longer therapy with perindopril would be required to obtain a more significant effect on plasma NO concentration.
The results obtained confirm the increase of oxidative stress and the dysfunction of vascular endothelium in elderly patients with hypertension. Taking into account that angiotensin convertase inhibitors belong to those medicines which, besides hypotensive actions, are widely regarded as having antioxidant properties, they should be widely used in elderly patients´ hypertension therapy.
Piśmiennictwo
1. Zalba G. et al.: Vascular oxidant stress: Molecular mechanisms and pathophysiological implications. J. Physiol. Biochem. 2000; 56:57-64. 2. Zalba G. et al.: Oxidative stress in arterial hypertension role of NAD(P)H oxidase. Hypertension 2001; 38:1395-9. 3. Sozmen B. et al.: Catalase paraoxonase in hypertensive types 2 diabetes mellitus: correlation with glycemic control. Clin. Biochem. 1999; 32(6):423-7. 4. Kumar K.V., Das U.N.: Are free radicals involved in the pathobiology of human essential hypertension? Free Radic. Res. Commun. 1993; 19(1):59-66. 5. Jana C.K. et al.: Specificity of age-related carbonylation of plasma proteins in the mouse and rat. Arch. Biochem. Biophys. 2002; 397(2):433-9. 6. Garibaldi S. et al.: Relationships between protein carbonyls, retinal and tocopherols level in human plasma. Biochem. Mol. Biol. Int. 1994; 34(4):729-36. 7. Chevion M. et al.: Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic. Res. 2000; 33:99-108. 8. Zalba G. et al.: Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelian dysfunction? Nephrol. Dial. Transplant. 2001; 16:2-5. 9. Vaziri N.D., Ding Y.: Effect of lead on nitric oxide synthase expression in coronary endothelial cells, role of superoxide. Hypertension 2001; 37:223-6. 10. O´Donell Freeman B.A.: Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ. Res. 2001; 88:12-21. 11. Romero J., Rackelhoff J.F.: Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999; 34(2):943-9. 12. Taddei S. et al.: Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001; 38:274-9. 13. Taddei S. et al.: Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation 1998; 97:2222-9. 14. Cominacini L. et al.: Antioxidant activity of different dihydropyridines. Biochim. Biophys. Res. Commun. 2003; 302:679-84. 15. Donmez G. et al.: The effects of losartan and enapril therapies on the levels of nitric oxide, malondialdehyde and glutathione in patients with essential hypertension. Jap. J. Physiol. 2002; 52:435-40. 16. Levine R.L. et al.: Determination of carbonyl content of oxidatively modified proteins. Methods Ensymol. 1990; 186:464-78. 17. Itzhaki R.F., Gill D.M.: Ilościowe oznaczanie białka metodą biuretową. Kłyszejko-Stefanowicz L., editors. Ćwiczenia z biochemii. PWN Warszawa 1999, p. 589-90. 18. Marletta M.A. et al.: Macrophage oxidation of L-arginine to nitrite and nitrate. Nitric oxide is an intermediate. Biochemistry 1998; 27:8706-11. 19. Goto S. et al.: Carbonylated proteins in ageing and exercise immunoblot approaches. Mech. Aging Dev. 1999; 107:245-53. 20. Agarwal S., Sohal R.S.: Relationship between ageing and susceptibility to protein oxidative damage. Biochem. Biophys. Res. Commun. 1993; 194(3):1203-6. 21. Romero J.C., Reckelhoff J.F.: Role of angiotensin and oxidative stress in essential hypertension. Hypertension 1999; 34:943-9. 22. Kędziora-Kornatowska K.: Effect of angiotensin convertase inhibitors and AT1 angiotensin receptor antagonists on the development of oxidative stress in the kidneys of diabetic rats. Clin. Chem. Acta 1999; 287:19-27. 23. De Caranagh E.M.V. et al.: Superoxide dismutase and glutathion peroxidase activites are increased by enalapril and captopril in mouse liver. FEBS Letters 1995; 361:22-4. 24. Node K. et al.: Reduced plasma concentrations of nitrogen oxide in individuals with essential hypertension. Hypertension 1997; 30:405-8. 25. Kumar K.V., Das U.N.: Effect of unsaturated fatty acid, prostaglandins, and free radicals on angiotensin converting enzyme activity in vitro. Proc. Soc. Exp. Biol. Med. 1997; 214:374-9. 26. Kohno M. et al.: Plasma levels of nitric oxide and related vasoactive factors following long-term treatment with angiotensin-converting enzyme inhibitor in patients with essential hypertension. Metabolism 1999; 48:1256-9.

