© Borgis - New Medicine 3/2013, s. 84-87
*Wiesława B. Duda-Król1, Małgorzata Kołodziejczak2, Iwona Sudoł-Szopińska2,3,4, Marlena Woch1, Michał Adamczyk5, Artur Mamcarz1
Atypical location of a stromal cell carcinoma in the rectal wall – a case report
1Third Clinic of Internal Medicine and Cardiology of the Medical University of Warsaw, Solec Hospital, Warsaw
Head of Clinic: Artur Mamcarz, MD, PhD
2General Surgery Department with Proctology Subdivision, Solec Hospital, Warsaw
Head of Department: Jacek Bierca, MD, PhD
Head of Proctology Subdivison: Małgorzata Kołodziejczak, MD, PhD
3Radiology Department, Institute of Rheumatology, Warsaw
Head of Department: prof. Sudoł-Szopińska, MD, PhD
4Department of Diagnostic Imaging, Warsaw Medical University
Head of Department: prof. Wiesław Jakubowski, MD, PhD
5Diagnostic Imaging Department, Central Clinical Hospital of the Ministry of Internal Affairs & Administration, Warsaw
Head of Department: prof. Jerzy Walecki, MD, PhD
Summary
Gastrointestinal stromal tumours (GIST) are rare proliferative lesions arising from the mesenchyme. In the United States, there are approximately 5000 cases diagnosed per year. The incidence of GISTs in males and females is equal. Only in about 5% of the cases, these lesions occur in the large intestine – whether in the submucosal, intramural or subserosal location. Approximately 10-30% of the GISTs have an asymptomatic course, and are incidentally diagnosed during imaging or surgical procedures performed for other indications. This article presents the case of a 74-year old female, who was found to have a stromal tumor in the wall of the rectum. The initial diagnosis was based on a proctological examination, anorectal endosonography and computed tomography. The final diagnosis was established based on histological analysis of material obtained via core-needle biopsy. The patient was referred to the Institute of Oncology, where she was qualified for neoadjuvant therapy, and later underwent surgery. A two-year observation period did not reveal any local recurrence or distant metastases.
GISTs occur very rarely in the rectum and thus pose a diagnostic challenge. Endosonography and computed tomography are useful in determining the location of the tumor with respect to the pelvic structures. The final diagnosis rests upon histopathological and immunohistochemical analysis of material obtained via core-needle biopsy.
Abbreviations: GIST = Gastrointestinal stromal tumors, BMI = body mass index, CT = computed tomography, MRI = magnetic resonance imaging
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are rare proliferative lesions arising from the mesenchyme, which constitute only 1-3% of primary malignant gastrointestinal tumors (1, 2). In the United States, there are approximately 5000 cases diagnosed per year, with an equal incidence among males and females (3-5). The peak in their incidence usually occurs in the 5th-7th decade of life, but the tumors can sporadically occur in younger patients (3, 6, 7). GISTs are most often located in the stomach (60-70%), small intestine (25-35%), rarely in the large intestine (5%), esophagus (2-3%), and sporadically in the omentum, mesentery, or in the retroperitoneal space (7-12). GISTs in the rectum have thus far been described in only a few articles (13-16).
GIST location may be submucosal, intramural or subserosal. In approximately 10-30% of cases the tumors are asymptomatic. They are characterized by local proliferation, which could lead to compression of the mucous membrane and trophic disturbances with possible necrosis and subsequent ulceration (3). GISTs most commonly metastasize to the liver, peritoneum; sporadically to the lungs, pleura, bones; and there are rare reports of brain metastases. The prognosis depends on the size of the tumor, its mitotic index (the ratio of the number of cells undergoing mitosis to the total number of cells), and the depth of its infiltration into the mucous membrane (3, 8-10).
CASE REPORT
A 74-year old female was admitted electively to the Third Clinic of Internal Medicine and Cardiology of the Medical University of Warsaw for diagnosis of a lesion detected in the rectal wall on rectal examination. The patient’s medical history revealed pain in the perineal and coccygeal area, unrelated to defecation, that had lasted for about 6 months prior to admission. Initially, this pain responded to oral nonsteroidal anti-inflammatory drugs (NSAIDs), but in the month prior to admission it increased in severity, and was accompanied by tenesmus. The patient denied weight loss and increased temperature; her stools were normal. The patient’s past medical history included peptic ulcer disease (for which she periodically took proton pump inhibitors (PPIs]), arterial hypertension (effectively controlled with an angiotensin receptor blocker (ARB) and a calcium channel blocker (CCB)), spondyloarthrosis, and smoking for many years.
On examination the patient was in overall good condition, BMI 25 kg/m2. The physical examination did not reveal any abnormalities. Laboratory tests, aside from leukocyturia with a negative urine culture, did not show any abnormalities. Tumor marker levels (CEA, AFP, and CA 125) were within normal limits. Gastroscopy showed gastritis with erosions and a focus of angiodysplasia in the duodenal bulb (ablated with Argon plasma coagulation). A rectal examination revealed a hard submucosal tumor, with a diameter of approximately 5 cm, on the left wall of the rectum, whose distal end reached approximately 2 cm from the anal margin. On anoscopy, stage I/II hemorrhoids were seen. Colonoscopy did not show any lesions in the colon, but the tumor which had been palpated on examination, measuring approximately 4 x 3 cm, was seen 2 cm above the anal sphincters, located on the left and partially also on the posterior walls of the rectum.
Initial diagnosis: a tumor of the rectal wall. Samples were taken for histopathology. The samples showed preserved glandular structure with a single lymph follicle and evidence of hyperemia and thickening of the muscularis mucosa. Immunohistochemical stains for CD 117 were negative.
The patient was referred for imaging studies. The abdominal ultrasound did not reveal any abnormalities. Anorectal endosonography showed a 41 x 38-mm
well-circumscribed hypoechoic lesion of heterogeneous echogenicity, with areas of necrosis, located intramurally in the postero-left-lateral wall, probably in the submucosal layer of the rectal wall. There was no perirectal lymphadenopathy (fig. 1A, B). A pelvic CT scan confirmed the presence of a tumor, approximately 4 x 4 x 5 cm in size, compressing the left side of the rectal lumen, located posteriorly to the vagina and to the left of the rectum, its distal end reaching the anus and protruding 2-2.5 cm distally into the perianal adipose tissue. The tumor exhibited slight contrast enhancement of its periphery, while its center contained an area of necrosis of 1.5 cm in diameter. The tumor was separated from the superior and middle parts of the vagina by a narrow strip of adipose tissue, while at its distal part, near the anus, no distinct boundary between the tumor, intestinal wall and pelvic diaphragm muscles was observed (fig. 2A, B, C). In addition, the abdominal CT scan showed a hepatic hemangioma with a diameter of 22 mm, and no other abnormalities of the abdominal organs. A transvaginal ultrasound did not show any pathology of the reproductive organs.
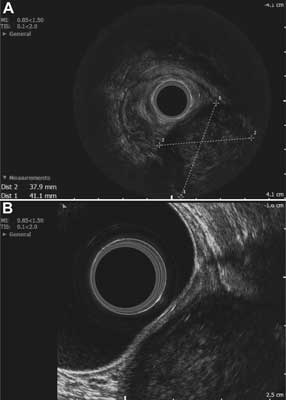
Fig. 1A, B. Endosonographic imaging: a hypoechogenic mass on the posterior-left-lateral wall of the rectum, with dimensions of 38 x 41 mm in the transverse plane, located intramurally, probably within the submucosal layer. A – Endosonographic imaging. B – Endosonographic imaging.
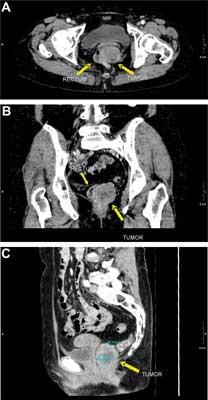
Fig. 2A, B, C. Computed tomography with contrast: (a) transverse (b) frontal and (c) sagittal cross-sections. The scans show the tumor located alongside the left side of the rectal wall, exhibiting non-homogeneous enhancement. The frontal plane image shows a partially indistinct border between the tumor and the rectal wall. A – Computed tomography with contrast: transverse. B – Computed tomography with contrast: frontal. C – Computed tomography with contrast: sagittal cross-sections.
The final diagnosis was based upon the histopathological examination of a core-needle biopsy sample.
The histopathological result showed the presence of spindle cell carcinoma with evidence of slight atypia, without mitotic figures. Alongside the immunohistochemical results – CD 117 (+++), CD 34 (+), DESM (+), SMA(+), S100 (-) – these findings were consistent with the diagnosis of a gastrointestinal stromal tumor (GIST).
Further treatment was continued at the Institute of Oncology, where the patient was qualified for neoadjuvant therapy (Dasatynib, which was administrated in a period of eight months) and later underwent surgery. A two-year observation period did not reveal any local recurrence or distant metastases.
DISCUSSION
GISTs are mesenchymal neoplasms originating from intramural ganglion cells of the myenteric (Auerbach’s) plexus (also known as interstitial Cajal’s cells which exhibit pacemaker activity, regulating the peristalsis of the gastrointestinal tract) or their precursors (2, 3, 17). For many years, these tumors were classified as various types of sarcomas, thus the existing epidemiological data is inaccurate. Only at the end of the 1990’s, with the progress of molecular biology and immunohistochemistry, was this group of stromal tumors placed in a separate category of mesenchymal-derived tumors, based on their immunohistochemical expression of antigens CD117 (in 95% of GIST cases) and CD34 (in 60-70% of cases) (3, 8-10). According to the current theory, GISTs arise from a neoplastic transformation of pluripotent stem cell precursors expressing the CD34 antigen, which differentiate towards pacemaker cells (2, 3). Approximately 10-30% of GISTs are completely asymptomatic, discovered incidentally during imaging or surgical procedures performed for other reasons (3). The symptoms are often non-specific: lack of appetite, weight loss, abdominal discomfort, abdominal pain, gastrointestinal bleeding, or gastrointestinal obstruction (3, 5). Similarly in the presented case, the reported symptoms were not specific and could have been due to the degenerative changes of the lower spine, or coccygodynia, as they were not related to bowel movements but rather to the patient’s position. The patient presented here did not lose weight nor did she report being subfebrile, which additionally confounded the diagnosis. The difficulties in diagnosing GISTs have also been emphasized by other authors (2, 18). These tumors may sometimes mimic reproductive tract lesions. Sometimes patients may be operated multiple times before the proper diagnosis is reached. The first diagnostic step in the presented case was the rectal examination, as the lesion was easier to detect on palpation than on visual inspection, due to its location beneath the rectal mucosa. Other reports of stromal tumors list CT scanning, MRI and endosonography as diagnostic methods of choice (19-24). In our patient, the endosonographic presentation of the tumor was quite distinct, whereas the CT image was not characteristic of GIST, as the greater part of the tumor appeared to be located outside the rectal wall, with the intramural origin of the lesion suggested only by a partially indistinct border between the tumor and the rectal wall. The diagnostic value of endosonography in such cases is also emphasized by other authors (1). The endosonographic examination enabled us to determine the location of the tumor with respect to particular layers of the rectal wall and to exclude the presence of abnormal perirectal lymph nodes. However, it did not allow for the differentiation of a stromal tumor from other mesenchymal-derived tumors (2). The final diagnosis was only possible on the basis of the histopathological and immunohistochemical assessments of the collected tissue sample (3). In the described patient, the conventionally collected samples proved to be insufficient to establish the diagnosis, probably due to overly superficial sampling of the rectal mucous membrane. The patient was referred to the Institute of Oncology, where a core-needle biopsy of the tumor was performed, upon which the final diagnosis was established. The patient was then qualified to receive neoadjuvant therapy (Dasatynib), and later operated. This treatment modality of stromal tumors is generally accepted by oncologists, due to the significant dimensions of the tumor at the time of diagnosis (25, 28). According to literature, surgical removal of GISTs is possible in only 50% of patients (29). In the presented case, the tumor was of large size, which prevented primary surgery. The use of neoadjuvant therapy led to a decrease in tumor mass, allowing for its subsequent surgical removal.
CONCLUSIONS
GISTs occur very rarely in the rectum and thus pose a diagnostic challenge. The endosonographic and CT examinations are useful in determining the tumor’s location relative to pelvic structures. The final diagnosis is possible on the basis of histopathological and immunohistochemical analysis of samples obtained via core-needle biopsy.
Piśmiennictwo
1. Berton F, Gola G, Wilson SR: Perspective on the Role of Transrectal and Transvaginal Sonography of Tumors of the Rectum and Anal Canal. Am J Rad 2008; 190: 1495-1504. 2. Zawisza A, Milewski J, Rydzewska G: Nowotwory stromalne przewodu pokarmowego. [W:] Wybrane zagadnienia gastroenterologii praktycznej. Małecka-Panas E, Rydzewska G (red.). Termedia Wydawnictwa Medyczne; Poznań 2007, 103-113. 3. Cichoż-Lach H, Kasztelan-Szczerbińska B, Słomka M: Stromalne guzy przewodu pokarmowego – epidemiologia, obraz kliniczny, diagnostyka, rokowanie oraz zasady leczenia. Pol Arch Med Wewn 2008; 118(4): 216-221. 4. Pietruszka M, Toczko Z: Olbrzymi guz podścieliskowy przewodu pokarmowego (GIST) – opis przypadku. Pol Przegl Chir 2006; 78: 51-58. 5. Prywiński S, Szopiński J, Wierzchowski P, Dąbrowicki S: Guz jelita cienkiego typu GIST jako przyczyna masywnego krwawienia do przewodu pokarmowego – opis przypadku. Chir Pol 2008; 10(2): 107-112. 6. Jaworski R, Zieliński J, Gross M, Kopacz A: Enormous gastrointestinal stromal tumor – a case report. J Onkol 2008; 58(1): 55-57. 7. Milnerowicz S, Strutyńska-Karpińska M, Markowska-Woyciechowska A, Rabczyński J. Guz stromalny przestrzeni zaotrzewnowej wywodzący się z dwunastnicy – przedstawienie przypadku. Adv Clin Exp Med 2005; 14(1): 175-178. 8. Miettinen M, Lasota J: Gastrointestinal Stromal Tumors (GISTs): Definition, Occurrence, Pathology, Differential Diagnosis and Molecular Genetics. Pol J Pathol 2003; 54(1): 3-24. 9. Miettinen M, Lasota J: Gastrointestinal Stromal Tumors: Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis. Arch Path Lab Med 2006; 130(10): 1466-1478. 10. Miettinen M, El-Rifai WE, Sobin LH, Lasota J: Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002; 33: 478-483. 11. Todoroki T, Sano T, Samurai S et al.: Primary omental Gastrointestinal stromal tumor (GIST). World J Surg Oncol 2007; 5: 66. 12. Zaręba K, Kamocki Z, Hołody-Zaręba J et al.: Rzadki przypadek współistnienia gruczolakoraka, raka endokrynnego i guza stromalnego żołądka; Przegl Gastro 2010; 5(5): 297-300. 13. Lau S, Lui CY, Yeung YP et al.: Gastrointestinal stromal tumor of rectum: a report of 2 cases. J Comput Assist Tomogr 2004; 27: 609 -615 14. Nasu K, Ueda T, Kai S et al. Gastrointestinal stromal tumor arising in the rectovaginal septum. Int J Gynecol Cancer 2004; 14: 373-377. 15. Tan GY, Chong CK, Eu KW, Tan PH: Gastrointestinal stromal tumor of the anus. Tech Coloproctol 2003, 7: 169-172. 16. Nigri GR, Dente M, Valabrega S et al.: Gastrointestinal stromal tumor of the anal canal: an unusual presentation. World J Surgl Oncol 2007; 5: 20. 17. Fletcher CD, Berman JJ, Corless C et al.: Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 2002; 10: 81-89. 18. Wroński M, Cebulski W, Pawłowski W, Krasnodębski IW: Trudności diagnostyczne u chorych z guzem stromalnym przewodu pokarmowego. Przegl Gastr 2006; 1(3): 115-120. 19. Panzironi G, Manganaro L, Ricci F et al.: A case of rectal GIST: findings of MR-spiral CT imaging and transrectal ultrasound guided biopsy. Eur J Rad Extra 2003; 47(2): 66-69. 20. Yeun Jung Lim, Hee Jung Son, Jong-Soo Lee et al.: Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol 2010; 16(4): 439-444. 21. Aydin A, Tekin F, Günşar F et al.: Value of endoscopic ultrasonography for upper gastrointestinal stromal tumors: a single center experience 2004; 15(4): 233-237. 22. Lau S, Tam KF, Kam CK et al.: Imaging of gastrointestinal stromal tumor (GIST). Clin Radiol 2004; 59: 487-498. 23. Chak A: EUS in submucosal tumors. Gastro Endosc 2002; 56 (Suppl. 4): S43-49. 24. Velasco S, Milin S, Maurel C et al.: Scanographic features of gastrointestinal stromal tumors. Gastro Clin Biol 2008; 32(12): 1001-1013. 25. Santos Fernandes G, Cutait de Castro Cotti G, Freitas D et al.: Downstaging of a rectal gastrointestinal stromal tumor by neoadjuvant imatinib therapy allowing for a conservative surgical approach. CLINICS 2009; 64(8): 819-821. 26. McAuliffe JC, Hunt KK, Lazar AJF et al.: A Randomized, Phase II Study of Preoperative plus Postoperative Imatinib in GIST: Evidence of Rapid Radiographic Response and Temporal Induction of Tumor Cell Apoptosis. Ann Surg Onkol 2009; 16: 910-919. 27. Nakamura T, Ihara A, Mitomi H et al.: Gastrointestinal Stromal Tumor of the Rectum Resected by Laparoscopic Surgery: Report of a Case. Surg Today 2007; 37: 1004-1008. 28. Ebihara Y, Okushiba S, Kawarada Y et al.: Neoadjuvant imatinib in a gastrointestinal stromal tumor of the rectum: Report of a case. Surg Today 2008; 38: 174-177. 29. DeMatteo RP, Lewis JJ, Leung D et al.: Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2002; 31: 51-58.

