© Borgis - New Medicine 1/2008, s. 13-15
*Jolanta Kłopocka
Flow cytometric analysis of eosin-5-maleimide binding to protein of band 3 and Rh-related proteins in diagnosis of hereditary spherocytosis
Department of Biophysics Medical Centre of Postgraduate Education Warsaw, Poland
Summary
Introduction
Flow cytometric analysis of eosin-5-maleimide (EMA) binding to red cells is a screening test for the diagnosis of hereditary spherocytosis (HS).
Interaction of EMA with the ĺ-NH2 group of lysine in band 3 protein was previously reported.
Aim
The aim of this work was to introduce modified flow cytometric analysis of eosin-5-maleimide binding to protein of band 3 and Rh-related proteins as the basis of a screening test in diagnosis of hereditary spherocytosis.
Materials and methods
Red cells from 12 patients with membrane deficiencies in HS (spectrin in 4 patients, band 3 in 3 patients, protein 4.2 in 2 patients and combined spectrin/ankyrin in 3 patients) and from 26 healthy volunteers.
Flow cytometric analysis of chemically modified EMA-labelled red cells.
Results
Fluorescence intensity of EMA-labelled HS red cells is sensitive to changes
in the relative amounts of band 3, Rh AG, CD 47 and Rh proteins.
Conclusions
Binding of EMA to the integral proteins (band 3, RhAG, CD 47 and Rh proteins) implicated in red cell cytoskeleton underpins the high specificity of the flow cytometric test for HS
Introduction
The biochemical basis of red cell membrane cytoskeletal defects associated with hereditary spherocytosis (HS) is heterogeneous and connected with deficiency of proteins such as spectrin, ankyrin, protein 4.2 and band 3 [1, 2, 3, 4, 5]. These proteins can be tested by the sodium dodecyl sulfate polyacrylamide gel electrophoresis method (SDS–PAGE) [6], which is currently the reference laboratory test for the identification of membrane protein deficiencies in HS.
The reaction between eosin-5-maleimide (EMA) and the ĺ-NH 2 group of Lys-430 on erythrocyte band 3 protein was first used as the basis for the flow cytometric analysis of band 3 in HS patients [7, 8, 9]. Eosin-5-maleimide binds stoichiometrically to band 3 on intact red cells. Thus, a reduction of fluorescence intensity indicates a quantitative reduction of erythrocyte band 3.
A decrease of fluorescence intensity was also detected with spectrin and protein 4.2 deficient cells. The inability to detect ankyrin-deficient HS red cells may suggest that either location of ankyrin within the cytoskeleton is distal to the EMA binding site of band 3 or that ankyrin is ´immobilized´ within the cytoskeleton.
Densitometric analysis of red cell membrane components after SDS-PAGE is also known to be insensitive to estimating the ankyrin content [6] because of the compensation effect produced by ankyrin content in reticulocytes and young red cells that are present in greater numbers in non-splenectomized HS patients. The gel method is strictly a quantitative technique. It lacks sensitivity in detecting a slight protein deficiency in mild HS samples containing a mixture of normal and abnormal erythrocyte proteins.
The dye method is more sensitive in detecting a mixed population of red cells in a blood sample because flow cytometry analyses fluorescence intensity of single cells (King 2000) [9]. The dye method is a reliable, rapid diagnostic test (2 h from sample collection to result) and can serve well as a screening test for the diagnosis of HS. In patients with typical features of HS, diagnosis of HS can be made if the fluorescence intensity of EMA-labelled red cells falls within the reference range for HS red cells [9].
Further investigations showed that EMA also reacts with the exofacial sulfhydryl group on intact red cells (heat labile reaction) [10, 11, 12, 13]. Chemical modification of EMA binding in the flow cytometric method, i.e. selectively blocking amine and sulfhydryl groups on red cells, was used for further investigation of EMA binding [11].
Materials and methods
Red cells from 12 patients with HS: 4 patients with spectrin deficiencies, 3 with band 3 deficiencies, 2 with protein 4.2 deficiencies and 3 with combined spectrin/ankyrin deficiencies. Red cells from 26 healthy volunteers as normal controls, 4 samples of Rh null red cells used as negative controls in EMA binding.
Flow cytometric analysis of EMA-labelled intact red cells from normal, Rh null and hereditary spherocytosis was performed.
Chemical modifications: N-ethylmaleimide (NEM) and acetic anhydride were used to block reactive sulfhydryl and amine groups, respectively, on intact red cells. Rh null red cells lack the two thiol-containing Rh proteins (Mr 30-32 kD); therefore they were used as a control for the absence of fluorescence in the 32 kD region.
Results
The average mean channel fluorescence (MCF) reading for normal red cell samples was 20% greater than that of Rh null red cell samples, whereas the fluorescence of HS red cells was 30% lower than that of normal red cells, confirming an earlier observation [9].
NEM treatment of normal red cells resulted in 25% decrease in EMA fluorescence, whereas this treatment only produced a small difference in fluorescence intensity between untreated and NEM-treated Rh null red cells.
This finding indicates that untreated Rh null red cells have few accessible exofacial sulfhydryl groups. A further 20% reduction of EMA fluorescence was detected in HS red cells after NEM treatment.
The abrogation of amine groups by acetic anhydride treatment produced a very large similar reduction of EMA binding to all the samples tested.
Sulfhydryl reactive molecules on normal red cells contributed to an estimated 20% of EMA fluorescence, and the remaining 80% of the EMA was bound to the free N-terminal and ĺ-NH2 group of lysine on band 3 protein.
Conclusions
Three molecules containing sulfhydryl groups were shown by the SDS-PAGE method, i.e. CD 47, Rh associated glycoprotein (Rh AG) and Rh blood group proteins [14,15,16,17]. It was confirmed that these three membrane proteins were lower in HS compared to normal red cells [9] using immunoprecipitation and specific monoclonal antibodies and antibody binding sites. Except for band 3 transmembrane protein, spectrin, ankyrin and protein 4.2 cannot interact directly with EMA because of location on the cytoplasmic side of the red cell membrane (Fig. 1). In three patients with HS with complete protein 4.2 deficiency secondary reduction of CD 47 was found. This finding shows that protein 4.2 possesses binding sites for CD 47 as well as for band 3.
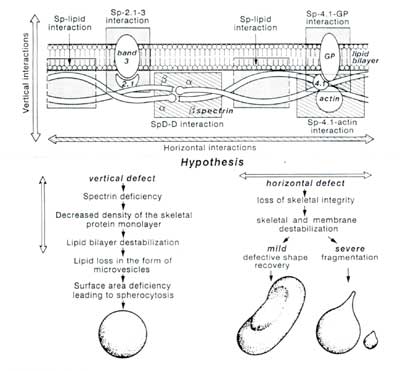
Fig. 1. Vertical and horizontal interactions of red cell membrane proteins [20].
Interaction of Rh protein complex with band 3 was shown in the case of complete band 3 deficiency (Bruce et. al., 2003) [18]. The conclusion from these studies is close proximity of the Rh related proteins to the vertical interaction involving â-spectrin, ankyrin and protein 4.2 with transmembrane band 3 protein. The partial reduction of Rh-related molecules to the maintenance of the integrity of spectrin-based red cell cytoskeleton remains to be elucidated.
The finding of reduced levels of Rh-related proteins in HS red cells correlates well with a previous report of Szymanski et al. [19], who detected a 50% reduction of blood group Rh (D) antigen sites in HS red cells. Their conclusion indicated no association between the blood group Rh genetics of families and the reduction of Rh (D) antigen sites in HS family members.
Binding of EMA to the integral proteins (band 3, Rh AG, CD 47 and Rh proteins) is the basis of the high specificity flow cytometric test for HS patients.
In conclusion, EMA binding screening tests [21, 22, 23] using intact red cells and SDS-PAGE of red cell membranes are distinct diagnostic tests that detect different sets of proteins that are deficient in HS red cells in patients with HS.
Piśmiennictwo
1. InoueT., Kanzi A., Kaku M., Yawata A., Takezono M., Okamoto N.,Wada H., SugiharaT., Yamada O., Katayama Y.and Nagata N.: Homozygous missense mutation (band 3 Fukuoka G 13OR) a mild form of hereditary spherocytosis with near - normal band 3 content and minimal change of membrane ultrastructure despite moderate protein 4.2 deficency. Br. J. Haematol., 1998, 102, 932-939. 2. Jarolim P., Palek J., Rubin H.L., Prchal J.T., Korsgen C. and Cohen C.M.: Band 3 Tuscaloosa Pro 327 Arg 327 substitution in the cytoplasmic domain of erythrocyte band 3 associated with spherocytic hemolytic anemia and partial deficiency of protein 4.2.Blood, 1992, 80, 523-529. 3. Jarolim P., Rubin H.L., Brabec V., Chrobak L., Zolotarev A.S., Alper S., Brugnara C.,Wichterle H. and Palek J.: Mutations of conserved arginines in membrane domain of erythroid band 3 lead to a decrease in membrane -associated band 3 and to the phenotype of hereditary spherocytosis. Blood, 1995, 85, 634-640. 4. Tse W.T. and Lux S.E.: Red blood cell membrane disorders. Br.J.Haematol., 1999, 104, 2-13. 5. Yawata Y.,Kanazaki A.,Yawata A.,Doerfler W.,Ozcan R. and Eber S.W.: Characteristic features of the genotype and phenotype of hereditary spherocytosis in the Japanese population. Int. J. Hematol., 2000, 71, 118-135. 6. Palek J. and Jarolim P.: Clinical expression and laboratory detection of red cell membrane protein mutations. Semin. Hematol., 1993, 30, 249-283. 7. Cherry R.J.: Rotational and lateral diffusion of membrane proteins. Biochim. Biophys. Acta, 1979, 559, 289-327. 8. Cobb C.E. and Beth A.H.: Identification of the eosinyl-5-maleimide reaction site on the human erythrocyte anion - exchange protein overlap with the reaction sites of other chemical probs. Biochemistry, 1990, 29, 8283-8290. 9. King M.J., Behrens J., Rogers C.A., Flynn C., Greenwood D. and Chambers K.: Rapid flow cytometric test for the diagnosis of membrane cytoskeleton-associated haemolytic anaemia.Br. J. Haematol., 2000, 111, 924-933. 10. Allard W.J.and Lienhard G. E.: Monoclonal antibodies to the glucose transporter from human erythrocytes.: Identification of the transporter as a M r = 55,OOO protein.J. Biol. Chem., 1985, 260, 8668-8675. 11. King M.J., Smythe J.S. and Mushens R.: Eosin-5-maleimide binding to band 3 and Rh-related proteins forms the basis of a screening test for hereditary spherocytosis. Br. J. Haematol., 2004, 124, 106-113. 12. Mallinson G., Martin P.G., Anstee D. J.Tanner M.J.A., Merry A.H., Tills D. and Sonneborn H.H.: Identification and partial characterization of the human erythrocyte membrane component (s) that express the antigens of the L W blood group system.: Biochem. J., 1986, 234, 649-652. 13. Majima E., Koike H., Hong Y.M., Shinohara Y. and Terada H.: Characterization of cysteine residues if mitochondrial ADP/ATP carrier with the -SH reagents eosin-5-maleimide and N-ethylmaleimide. J. Biol. Chem., 1993, 268, 22184-22187. 14. Cartron J.P.: Rh blood group system and molecular basis of Rh deficiency.Bailliere"s Clin. Haematol., 1999, 12, 655-689. 15. King M.J. and. Rogers C.A.: Involvement of the Rh complex and CD 47 in hereditary spherocytosis, Br. J. Hematol., 2001, 113, (Suppl 1), 61 (abstract). 16. Mawby W.J., Holmes C.H., Anstee D.J., Spring F.A. and Tanner M.J.A.: Isolation and characterization of CD 47 glycoprotein: a multispanning membrane protein which is the same as integrin - associated protein (IAP) and the ovarian tumour marker OA 3. Biochem. J., 1994, 304, 525-530. 17. Moore S., Woodrow C.E. and Mc Cleeland D.B.L.: Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy a. Nature, London, 1982, 295, 529-531. 18. Bruce J.L., Beckmann R., Ribero M.L., Peters L.L., Chasis J.A. at al.: A band 3 - based macrocomplex of integral and peripheral proteins in the RBC membranes, Blood, 2003, 101, 4180-4188. 19. Szymanski I.O., Araszkiewicz P., Odgren P., and Snyder L.M.: Decreased amount of the Rh antigen D in hereditary spherocytosis (H.S.). Br.J.Haematol., 1989, 73, 537-540. 20. Tanner M.J.A.: Red cell membrane structure and function: an overview. Brit. J. Haemat., 1992, 82, 257. 21. Cooper S., Zarkos K., Jurinkulravanish T., Dolatin M.,Brown R.:Diagnosis of hereditary spherocytosis by flow cytometric detection of eosin-5-maleimide binding to band 3, Australian J.Med.Sci., 2007,28,3,109-11. 22. Stoya G., Gruhn B., Vogelsang H., Baumann E., Linss W.: Flow cytometry as a diagnostic tool for hereditary spherocytosis. Acta Haematol., 2006, 116 (3), 186-191. 23. Kedar P.S., Colah R.B., Kulkarni S., Ghosh K., Mohanty D.: Experience with eosin-5-maleimide as a diagnostic tool for red cell membrane cytoskeleton disorders. Clin. Lab.Haematol., 2003,25(6),373-6.
