Tilden D1, Aristides M1, Stynes G1, Orlewska E2, Krzakowski M3, Jassem J4, Załuski J5, Włodarczyk H5
Cost-effectiveness of gemcitabine in combination with cisplatin versus vinorelbine in combination with cisplatin in the treatment of non-small cell lung cancer in Poland: a retrospective economic analysis of clinical trials
1M-TAG Hammersmith, United Kingdom
2Centre for Pharmacoeconomics, Warsaw, Poland
3Oncology Centre – M. Skłodowska-Curie Institute, Department of Lung and Thorax Cancer, Warsaw, Poland
4Department of Oncology and Radiotherapy, Medical University of Gdansk, Gdansk, Poland
5Great Poland Center of Cancer, Department of Chemotherapy, Poznan, Poland
Summary
Objective: To evaluate the cost-effectiveness of gemcitabine/cisplatin (Gem/Cis) vs vinorelbine/cisplatin combinations (Vin/Cis) in the treatment of non small cell lung cancer (NSCLC) in Poland.
Methods: Costs and effectiveness of Gem/Cis and Vin/Cis were based on resource and outcome data from published head-to-head clinical trials and local data on health-care resource utilisation and unit cost. Only direct medical costs resulting from the chemotherapy (acquisition and administration), concomitant medication and treatment of adverse events were assessed. Information on current treatment practice was obtained from the Polish oncologist expert panel. The perspective of health-care payers and time horizon of 1 year was considered. The one-way sensitivity analyses were performed.
Results: The results of clinical trials show no statistically significant differences between Gem/Cis and Vin/Cis in terms of overall survival. The total direct medical cost was 17 132 PLN and 17 023 for Gem/Cis and Vin/Cis respectively. Chemo-therapy acquisition cost was the major cost factor and was accounted for 64% and 50% of total cost for Gem/Cis and Vin/Cis respectively. The higher acquisition costs of Gem/Cis are offset by lower drug administration costs and lower rates of hospitalisation for Gem/Cis patients. The results of sensitivity analyses show that, in most circumstances, the Gem/Cis combination is broadly similar in cost to Vin/Cis.
Conslusion: Given the proven equal efficacy in treatment, and the higher levels of toxicity associated with the vinorelbine regimen, the analysis supports a cost-effectiveness argument for the use of gemcitabine in the treatment of advanced NSCLC.

INTRODUCTION
The treatment of non-small cell lung cancer (NSCLC) remains a difficult and controversial area in oncology. It is particularly appropriate to address topics in lung cancer in that this malignancy continues to be a major international health problem, is the leading cause of cancer related death in many countries, and its incidence is rising in most countries. This study will consider issues in the treatment of patients with advanced lung cancer, a group that includes the majority of patients with this disease. As the use of palliative chemotherapy increases, the costs of cancer chemotherapy will escalate, and it is important to understand how these costs relate to benefits.
This paper presents an adaptation of the economic evaluation performed for the National Institute of Clinical Excellence (NICE) in England and Wales. The focus of the report is on the comparison between gemcitabine/cisplatin and vinorelbine/cisplatin in the Polish setting. These represent the new wave of therapies used in NSCLC. Until now, there has been little European evidence on the relative cost-effectiveness of these new regimens.
EPIDEMIOLOGY
Lung cancer is in Poland the most prevalent malignant tumor among men (29.4%) and among women remains the second localisation (7%) (1-3). There are more than 20 000 new cases annually (4). Lung cancer remains the leading cause of cancer-related mortality in both sexes, accounting for every third and every tenth of cancer death in Polish men and women, respectively. Approximately 80% of lung cancers are of the non-small-cell (NSCLC) histology (4). At the time of diagnosis 50-55% of patients have advanced or metastatic disease (5). The long term prognosis is poor: the median survival of patients with untreated NSCLC is only four-five months, with survival rate at one year of only 10% (6).
THE ROLE OF CHEMOTHERAPY IN THE TREATMENT OF NSCLC
The evidence from randomized clinical trials showed conclusively that administration of chemotherapy offers a significant, but modest, survival advantage for all stages of NSCLC (6-9). In early-stage disease, postoperative cisplatin-based adjuvant chemotherapy is associated with a hazards ratio of 0.87, which is equivalent to an absolute survival benefit of 5% at 5 years. For patients with more advanced tumors, the hazards ratio for chemotherapy is 0.73, with 10% absolute improvement in survival at 1 year over supportive care alone.
In addition randomized studies comparing chemotherapy with the best supportive care have shown that chemotherapy reduces symptoms and improves the quality of life (10, 11).
A number of chemotherapy agents have been shown to be active in NSCLC (4, 12, 13). There is quite a widespread agreement about the inclusion of cisplatin in any first-line chemotherapy combination (8, 9). However, although cisplatin remains an important component of the most active regimens, paradoxically it may be this agent that limits the use of chemotherapy in NSCLC. In the last decade, the introduction into clinical practice of several new drugs with proven antitumor activity in NSCLC patients and reduced toxicity has renewed the interest of clinical oncologists in the treatment of this disease. Among newer active drugs, vinorelbine, gemcitabine and taxanes are the most promising, in view of their intrinsic cytotoxic activity and of their nonoverlapping toxicity and potential synergism when combined with cisplatin (13). The results obtained in phase II and III studies (14-42) showed that in advanced NSCLC:
1) the addition of a new agent to cisplatin improves both survival and quality of life when compared with cisplatin alone,
2) new agents in monotherapy demonstrate similar activity as standard cisplatin-based combination chemotherapy,
3) new agents in combinations with cisplatin (third generation regimens) provide at least significantly higher response rate and longer time to disease progression when compared with standard cisplatin-based combination chemotherapy,
4) there are no significant differences in survival among patients who received various third-generation regimens in head-to-head studies. However, there are differences in toxicity profile among treatment groups.
CLINICAL NEED AND PRACTICE
Most patients with NSCLC in Poland receive BSC and palliative radiotherapy. This may be due, in part, to a perception amongst health professionals and patients that chemotherapy is toxic and ineffective for the treatment of NSCLC. It is estimated that in Poland 4000-5000 patients diagnosed with NSCLC are potential candidates for chemotherapy (4). The most frequently used chemotherapy regimens are "standard” cisplatin-based regimens such as PE (etoposide/cisplatine), MIC (mitomycin, ifosfamide, cisplatin) and MVP (mitomycin, vindesine or vinblastine and cisplatin) (4).
According to clinical guidelines gemcitabine, vinorelbine and paclitaxel each all combined with a platinum analogue can be considered as part of first-line chemotherapy options for advanced (stage III and IV) NSCLC patients. Docetaxel monotherapy should be considered as a second line treatment in patients with locally advanced or metastatic NSCLC.
THE AIM OF THE STUDY
The aim of the study was to evaluate the cost-effectiveness of gemcitabine in combination with cisplatin (GemCis) versus vinorelbine with cisplatin (VinCis) in the treatment of patients with NSCLC stage IIIB and IV in the Polish setting. Vinorelbine/cisplatin regimen is in Poland the most commonly used novel cisplatin based doublet in NSCLC.
METHODS
The analysis builds on the foundations of the original analysis to NICE (National Institute of Clinical Excellence). The main modifications are adjustments for the costs of chemotherapy drug acquisition and administration costs, and inclusion of costs for a wider range of hospitalisations due to adverse events. There are also adjustments to the unit costs, and to resource use associated with radiotherapy and concomitant medications.
Data sources
Data on health outcome (expected survival), adverse event rates, specification for each regimen and the number of treatment cycles derived from the published head-to-head clinical trial (41). This trial randomised NSCLC patients to gemcitabine/cisplatin or vinorelbine/cisplatin and the interim analysis was carried out when 60 patients per arm were evaluable for survival. All patients had locally advanced or metastatic NSCLC (42% stage IIIB, 58% stage IV), were aged =70 years and had performance status =1 (41).
Where utilisation of particular health care resources was not available from the clinical trial report, values were derived from a search of the literature. The literature providing additional resource utilisation data included approved product information, Scagliotti et al. (42) (a clinical trial comparing gemcitabine/cisplatin, paclitaxel/carboplatin and vinorelbine/cisplatin), Schiller et al. (43) (a clinical trial comparing gemcitabine/cisplatin, paclitaxel/carboplatin, paclitaxel/cisplatin and docetaxel/cisplatin) and Rubio Terres et al. (44) (an unpublished modelled evaluation of gemcitabine/cisplatin compared with paclitaxel/carboplatin and vinorelbine/cisplatin).
Data on health-care utilization associated with country-specific treatment patterns were obtained from the panel of clinical experts through a survey performed in 3 Polish oncological centers (in Warsaw, Poznań and Gdańsk), which manage above 50% of patients in Poland under treatment for NSCLC. The questionnaire collected information on current treatment practices were ratified at the expert panel meeting.
Study design
The analysis was developed from payer´s perspective. Only direct health care costs are included in each analysis. Therefore, costs associated with lost time at paid work, at unpaid work, and lost leisure activities for patients and carers are excluded from the analyses. Costs associated with social services in support care are also excluded. Any potential for reducing such costs due to superior treatment of advanced NSCLC should be considered qualitatively, even if costs are not quantified.
An intention-to-treat approach to costing has been adopted, where resources used to treat eligible patients randomised to study therapy are valued. In this way, any potential inter-relationships between resource use and outcomes are accounted for. In each evaluation of gemcitabine/cisplatin, costs of chemotherapy drugs and administration are assumed to be incurred within an initial 12-month period and therefore remain undiscounted. All other costs also remain undiscounted, as it is difficult to ascertain which costs are incurred beyond the first year. The absence of discounting is unlikely to make a substantial difference, as the most significant cost components (chemotherapy, other drugs, hospitalisation) are all incurred in the initial 12 months. All costs were calculated in 2002 values.
Dosage regimens for the two therapies are shown in table 1.
Table 1. Chemotherapy regimens used in the reference clinical trial (41).
| GEM/CIS combination | Gemcitabine | Cisplatin |
| Protocolled dose | 1000 mg/m2 | 100 mg/m2 |
| Dispensed dosea | 1800 mg | 180 mg |
| Doses per cycle | 3 | 1 |
| Cycles per patientb | 2.6 | 2.6 |
| Length of cycle | 28 days | 28 days |
| Day(s) of administration | 1, 8, 15 | 2 |
| VIN/CIS combination | Vinorelbine | Cisplatin |
| Protocolled dose | 30 mg/m2 | 120 mg/m2 |
| Dispensed dosea | 54 mg | 216 mg |
| Doses per cycle | 5 | 1 |
| Cycles per patientb | 2.5 | 2.5 |
| Length of cycle | 35 days | 35 days |
| Day(s) of administration | 1, 8, 15, 22, 29 | 1, 29, then every subsequent 42 days |
a Dispensed doses assume a mean body surface area (BSA) of 1.8 m2. This is consistent with the BSA of patients enrolled in the clinical trial
b Average number of cycles per patient as reported in the clinical trial
RESULTS
Health outcomes
Health outcomes are summarised in Figure 1. Slightly greater values were observed with gemcitabine/cisplatin compared with vinorelbine/cisplatin in the median survival time (42 weeks versus 35 weeks) and response rates (30% versus 25%). Significance testing was not conducted on these interim data but it is noteworthy that the vinorelbin/cisplatin arm was stopped due to an early stopping rule for unfavourable survival. As statistical significance of the difference was not reported for efficacy end-points, the conservative assumption of equal efficacy across treatment arms was made. The economic evaluation based upon the Comella et al.trial (41) thus takes the form of a cost-minimisation analysis.
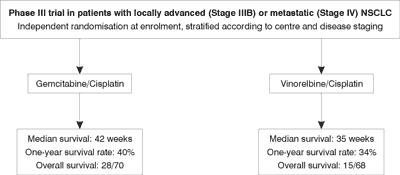
Figure 1. Treatment groups and health outcomes in the reference clinical trial (41).
Costs
A comprehensive set of direct health care costs was included in these evaluations. This was composed of:
l Acquisition of the chemotherapy
l Administration of the chemotherapy
l Hospitalisations associated with adverse events (febrile neutropenia, thrombocytopenia, anaemia, nausea and vomiting, neuropathy and events requiring IV antibiotics)
l Costs of other medical resources (visits to health care professionals, radiotherapy, transfusions of blood products and the use of concomitant medications)
Costing of chemotherapy drug acquisition
Acquisition of chemotherapy is based on the expected drug cost per cycle of chemotherapy multiplied by the average number of cycles administered as reported in the respective trial (tab. 2).
Table 2. Number and cost of chemotherapy vials used in the Comella et al. trial (41).
| Regimen | Regimen cost per patienta (PLN) | Drug | Drug cost per patient (PLN) | Size of vial | Number of vials per patient | Unit cost per vial (PLN) |
| GEM/CIS | 10975.77 | Gemcitabine | 10790.91 | 1000 mg
200 mg | 7.8
31.2 | 756.85
156.65 |
| Cisplatin | 184.86 | 50 mg
25 mg
10 mg | 7.8
2.6
2.6 | 18.20
11.20
5.30 |
| VIN/CIS | 8558.50 | Vinorelbine | 8350.00 | 50 mg
10 mg | 12.5
12.5 | 550.00
118.00 |
| Cisplatin | 208.50 | 50 mg
10 mg | 10
5 | 18.20
5.30 |
a Regimen cost per patient = sum (type and size of vial x unit cost per vial)
Costing of chemotherapy administration
Cost of chemotherapy administration (tab. 3) was calculated by multiplying expected number of total doses administered by the unit cost per drug administration (the unit cost per drug administration is weighted by the expected number of inpatient and outpatient administrations).
Table 3. Number and cost of chemotherapy administrations in the Comella et al. trial (41).
| Regimen | Admin. cost per patienta (PLN) | Admin. days per cycle | Cycles per patient | Admin. days per patient | IP admin. daysb | OP admin. daysc | OP admin. cost per day (PLN) |
| GEM/CIS | 1287.00 | 3 | 2.6 | 7.8 | 0 | 7.8 | 165.00 |
| VIN/CIS | 2062.50 | 5 | 2.5 | 12.5 | 0 | 12.5 | 165.00 |
a Admin. cost per patient = (inpatient admin. days x unit cost of inpatient admin days) + (outpatient admin. days x unit cost of outpatient admin days)
b IP: Inpatient
c OP: Outpatient
Costing of hospitalisation due to adverse events
The cost of hospitalization due to adverse events was calculated by multiplying the expected number of patients hospitalised with an adverse event as reported by the respective clinical trial (or other source as appropriate) by the expected cost per hospital episode (tab. 4.)
Table 4. Percentage of patients hospitalised for each adverse event and the cost of hospitalisations.
| Adverse event | Unit cost (PLN) | Gemcitabine/Cisplatin | Vinorelbine/cisplatin |
| Percentage | Cost (PLN) | Percentage | Cost (PLN) |
| Febrile neutropenia | 4142.00 | 10.7%a | 442.37 | 27.8%b | 1151.48 |
| Thrombocytopenia | 1326.00 | 30.0%c | 397.80 | 20.0%c | 265.20 |
| Nausea/vomiting | 613.00 | 30.0%c | 183.90 | 50.0%c | 306.50 |
| Neuropathy | 2321.00 | 3.0%f | 69.63 | 20.0%g | 464.20 |
| Anaemia | 831.00 | 1.7%a | 14.13 | 3.4%d | 28.25 |
| IV antibiotics | 1454.00 | 25.0%a | 363.65 | 26.5%e | 385.93 |
| Hospitalisation cost/patienth | | | 1471.47 | | 2601.56 |
a Schiller et al. (42)
b Product information
c Comella et al. (41)
d Scagliotti et al. (43)
e Average of other novel regimens
f Average of Comella et al. (41), Rubio Terres et al. (44), Schiller et al. (42) results
g Average of Rubio Terres et al. (44), Schiller et al. (42) results
h Hospitalisation cost per patient = sum (probability of suffering each adverse event x unit cost of each adverse event)
Cost of other medical resources
Cost of other medical resources includes visits to health care professionals (tab. 5), radiotherapy (tab. 6), transfusions of blood and blood products (tab. 7) and the use of concomitant medications (tab. 8).
Table 5. Cost of GP visits for patients receiving chemotherapy for advanced NSCLC in the Comella et al. (41).
| Gemcitabine/Cisplatin | Vinorelbine/cisplatin |
| Time to progression | 4.5 months | 4.0 months |
| Overall survival | 8.1 months | 8.1 months |
| Number of GP visits | 11.7 | 12.2 |
| Cost per visit | PLN 35.00 | PLN 35.00 |
| Cost of GP visitsa | PLN 409.50 | PLN 427.00 |
a Cost of GP visits per patient = assumed number of visits x unit cost per visit
Table 6. Cost of patients requiring radiotherapy.
| Gemcitabine/cisplatin | Vinorelbine/Cisplatin |
| Percentage of patients requiring radiotherapy | 23.3%b | 23.3%b |
| Administration cost of radiotherapy | PLN 7717.00 | PLN 7717.00 |
| Cost of radiotherapya | PLN 1800.63 | PLN 1800.63 |
a Cost of radiotherapy per patient = probability of requiring radiotherapy x administration cost of radiotherapy
b Comella et al. (41)
Table 7. Cost of blood product transfusions over the course of therapy (41). Unit costs of blood products can be found in the table in the appendix.
| Gemcitabine/cisplatin | Vinorelbine/Cisplatin |
| Packed red blood cells | 16%a | 21%a |
| Cost per patientb | PLN 212.00 | PLN 212.00 |
| Platelets | 8%a | 8%a |
| Cost per patientc | PLN 372.00 | PLN 372.00 |
| Expected cost per patientd | PLN 63.68 | PLN 74.28 |
a Scagliotti et al. (43)
b A cost of PLN 212.00 is based on a 600 ml transfusion (PLN 100.00 per 300 ml) and a cost to perform the transfusion of PLN 12.00
c A cost of PLN 372.00 is based on a 300 ml transfusion (PLN 60.00 per 50 ml) and a cost to perform the transfusion of PLN 12.00
d Cost of blood transfusions per patient = (probability of requiring packed red blood cells x cost of cells per patient) + (probability of requiring platelets x cost of platelets per patient)
Table 8. Units and costs of concomitant medications in patients receiving novel chemotherapy for NSCLC in the Comella et al. (41). Unit costs of concomitant medications can be found in the table in the appendix.
| Medication | Gemcitabine/Cisplatin | Vinorelbine/cisplatin |
| Tropisetron (5 mg infusion followed by 5 mg daily for 5 days) |
| 5 mg vials | 3 | 4 |
| 5 mg tablets | 15 | 20 |
| Cost (tropisetron) | PLN 1045.32 | PLN 1393.76 |
| Saline (units) | 12351 | 17563 |
| Cost (saline) | PLN 53.36 | PLN 75.87 |
| Chlorpromazine HCl (10 mg, 6 daily, 5 days) |
| Units per patient | 90 | 120 |
| Cost (chlorpromazine) | PLN 9.00 | PLN 12.00 |
| Laxatives |
| Units per patient | 30 | 30 |
| Cost (laxatives) | PLN 16.50 | PLN 16.50 |
| Total pre/post medsa | PLN 1124.18 | PLN 1498.13 |
a Cost of concomitant medication per patient = sum (expected unit requirement of each medication x unit cost of each medication)
In the comparisons of gemcitabine/cisplatin with vinorelbine/cisplatin the visits to medical professionals were not reported. It was assumed the costs for visits to nurses and other professionals (excluding GPs) were identical between the treatment groups. On the basis of opinion of Polish oncology experts it is assumed that patients will visit a GP once monthly during treatment (in addition to all the chemotherapy and associated visits to oncologists), and twice monthly for palliative therapy – symptomatic control and palliation – after disease progression.
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Zatoński W, Przewoźniak K. Palenie tytoniu w Polsce: postawy, następstwa zdrowotne i profilaktyka. Centrum Onkologii-Instytut im. M. Skłodowskiej Curie, Warszawa 1996.
2. Zatoński W. Rozwój sytuacji zdrowotnej w Polsce na tle innych krajów Europy Środkowej i Wschodniej. Wydawnictwo ANTA Warszawa 2000.
3. Zatoński W, Tyczyński J (red.). Epidemiologia nowotworów złośliwych w Polsce w piętnastoleciu 1980-1994. Centrum Onkologii-Instytut im. M. Skłodowskiej Curie, Warszawa 1997.
4. Krzakowski M, Siedlecki P. Standardy leczenia systemowego nowotworów złośliwych u dorosłych w Polsce. Polskie Towarzystwo Onkologii Klinicznej, Warszawa, 1999.
5. Krzakowski M (red). Onkologia Kliniczna. Borgis, Warszawa 2001.
6. Rapp E, Pater JL, Willan A, et al. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer - a report of a Canadian multicenter randomized trial. J Clin Oncol 1998; 6: 633-41.
7. Marino P, Pampallona S, Preatoni A, et al. Chemotherapy vs. supportive care in advanced non-small-cell lung cancer: results of a meta-analysis of the literature. Chest 1994; 106: 861-5.
8. Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311: 899-909.
9. Non-Small Cell Lung Cancer Collaborative Group: Chemotherapy for non-small cell lung cancer (Cochrane Review). Cochrane Library 2. Oxford, United Kongdom, The Cochrane Collaboration, 2000 (update software).
10. Cullen M, Oxman AD, Billingham J, Woodraffe C, at al. Mitomycin, ifoisfamide and cisplatin in unresectable non-small cell lung cancer: effects on survival and quality of life. J Clin Oncol 1999; 17:3188-94.
11. Helsing M, Bergman B, Thaning L, et al. Quality of life and survival in patients with advanced non-small cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposoide or supportive care only: A multicentre randomized phase III trial - Joint Lung Cancer Study Group. Eur J cancer 1998; 34: 1036-1044.
12. Gralla R. New directions in non-small cell lung cancer. Semin Oncol 1990; 17 (suppl.7): 20-29.
13. Lilelbaum RC, Green MR. Novel chemotherapeutic agents in the treatment of non-small cell lung cancer. J Clin Oncol 1993; 11: 1391-1402.
14. Fossella, F. V., Devore, R., Kerr, R. N., Crawford, J., Natale, M., Dunphy, F.,Kalman, L. A., Miller, V., Soo Lee, J., Moore, M., Gandara, D. R., Karp, D., Vokes,E. E., Kris, M. G., Kim, Y., Gamza, F., Hammershaimb, L., and TAX 320 Non-Small-Cell Lung Cancer Study Group. Randomised phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimes. J.Clin.Oncol.18(12), 2354-2362. 2000.
15. Bokkel-Huinink WW, Bergman B, Chemaissani A, Dornoff W, Drings P,Kellokumpu-Lehtinen PL et al. Single-agent gemcitabine: an active and better tolerated alternative to standard cisplatin-based chemotherapy in locally advanced or metastatic non-small cell lung cancer. Lung Cancer 1999;26:85-94.
16. Manegold C, Bergman B, Chemaissani A, Dornoff W, Drings P, Kellokumpu LP et al. Single-agent gemcitabine versus cisplatin-etoposide: early results of a randomized phase II study in locally advanced or metastatic non-small-cell lung cancer. Ann.Oncol. 1997;8:525-9.
17. Cardenal F, Lopez-Cabrerizo MP, Anton A, Alberola V, Massuti B, Carrato A et al. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J.Clin.Oncol.1999;17:12-8.
18. Crino L, Scagliotti GV, Ricci S, De Marinis F, Rinaldi M, Gridelli C et al.Gemcitabine and cisplatin versus mitomycin, ifosfamide, and cisplatin in advanced non-small-cell lung cancer: A randomized phase III study of the Italian Lung CancerProject. J.Clin.Oncol. 1999;17:3522-30.
19. Perng RP, Chen YM, Ming LJ, Tsai CM, Lin WC, Yang KY et al. Gemcitabine versus the combination of cisplatin and etoposide in patients with inoperable non-small-cell lung cancer in a phase II randomized study. J.Clin.Oncol. 1997;15:2097-102.
20. Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with 178 locally advanced or metastatic non-small-cell lung cancer. J.Clin.Oncol. 2000;18:122-30.
21. Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J.Clin.Oncol.2000;18:623-31.
22. Chang AY, Kim K, Glick J, Anderson T, Karp D, Johnson D. Phase II stud y of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: The Eastern Cooperative Oncology Group Results J.Natl.Cancer Inst.1993;85:388-94.
23. Ranson, M., Davidson, N., Nicolson, M., Falk, S., Carmichael, J., Lopez, P.,Anderson, H., Gustafson, N., Jeynes, A., Gallant, G., Washington, T., and Thatcher,N. Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small cell lung cancer. J.Natl.Cancer Inst. 92(13), 1074-1080. 2000.
24. Postmus PE, Giaccone G, Debruyne C, Sahmoud T, Splinter TA, van Zandwijk N. Results of the phase II EORTC study comparing paclitaxel/cisplatin with teniposide/cisplatin in patients with non-small cell lung cancer. EORTC Lung CancerCooperative Group. Semin.Oncol. 1996;23:10-3.
25. Gatzemeier, U., von Pawel, J., Gottfried, M., ten Velde, GPM, Mattson, K,DeMarinis, F, Harper, P, Salvati, F, Robinet, G, Lucenti, A, Bogaerts, J, and Gallant,G. Phase III comparative study of high-dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancer.J.Clin.Oncol. 18(19), 3390-3399. 2000.
26. Giaccone G, Splinter TA, Debruyne C, Kho GS, Lianes P, van Zandwijk N et al. Randomized study of paclitaxel-cisplatin versus cisplatin- teniposide in patients with advanced non-small-cell lung cancer. The European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group J.Clin.Oncol.1998;16:2133-41.
27. Baldini E, Tibaldi C, Ardizzoni A, Salvati F, Antilli A, Portalone L et al. Cisplatin-vindesine-mitomycin (MVP) vs cisplatin-ifosfamide-vinorelbine (PIN) vs carboplatin vinorelbine(CaN) in patients with advanced non-small- cell lung cancer (NSCLC): a FONICAP randomized phase II study. Italian Lung Cancer Task Force (FONICAP).Br.J.Cancer 1998;77:2367-70.
28. Colleoni M, Vicario G, Pancheri F, Sgarbossa G, Nelli P, Manente P. A randomized phase II trial of cisplatinum plus mitomycin-C plus vinorelbine and carboplatin plus vinorelbine in advanced non-small cell lung cancer. Int.J.Oncol. 1997;10:619-22.
29. Colucci G, Gebbia V, Galetta D, Riccardi F, Cariello S, Gebbia N. Cisplatin and vinorelbine followed by ifosfamide plus epirubicin vs the opposite sequence in179advanced unresectable stage III and metastatic stage IV non-small- cell lung cancer: a prospective randomized study of the Southern Italy Oncology Group (GOIM).Br.J.Cancer 1997; 76:1509-17.
30. Comella P, Frasci G, De Cataldis G, Panza N, Cioffi R, Curcio C et al.Cisplatin/carboplatin + etoposide + vinorelbine in advanced non-small-cell lung cancer: a multicentre randomised trial. Gruppo Oncologico Campano. Br.J.Cancer1996;74: 1805-11.
31. Crawford J, O´Rourke M, Schiller JH, Spiridonidis CH, Yanovich S, Ozer H et al. Randomized trial of vinorelbine compared with fluorouracil plus leucovorin inpatients with stage IV non-small-cell lung cancer. J.Clin.Oncol. 1996;14:2774-84.
32. Depierre A, Chastang C, Quoix E, Lebeau B, Blanchon F, Paillot N et al. Vinorelbine versus vinorelbine plus cisplatin in advanced non-small cell lung cancer: arandomized trial. Ann.Oncol. 1994;5:37-42.
33. Furuse K, Fukuoka M, Kuba M, Yamori S, Nakai Y, Negoro S et al. Randomized study of vinorelbine (VRB) versus vindesine (VDS) in previously untreated stage IIIBor IV non-small-cell lung cancer (NSCLC). The Japan Vinorelbine Lung Cancer Cooperative Study Group. Ann.Oncol. 1996;7:815-20.
34. Le Chevalier T, Brisgand D, Douillard JY, Pujol JL, Alberola V, Monnier A et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non-small-cell lung cancer: results of a European multicenter trial including 612 patients. J.Clin.Oncol. 1994;12:360-7.
35. Lorusso V, Pezzella G, Catino AM, Guida M, Scoditti S, Lorenzo R et al. Results of a clinical multicentric randomized phase II study of non-small cell lung cancer treated with vinorelbine-cisplatin versus vinorelbine alone. Int.J.Oncol. 1995;6:65-8.
36. Martoni A, Guaraldi M, Piana E, Strocchi E, Petralia A, Busutti L et al. Multicenter randomized clinical trial on high-dose epirubicin plus cisplatinum versus vinorelbine plus cisplatinum in advanced non small cell lung cancer. Lung Cancer 1998; 22:31-8.
37. Perol M, Guerin JC, Thomas P, Poirier R, Carles P, Robinet G et al. Multicenter randomized trial comparing cisplatin-mitomycin- vinorelbine versus cisplatin- mitomycin-vindesine in advanced non-small cell lung cancer. "Groupe Francais de Pneumo-Cancerologie". Lung Cancer 1996;14:119-34.
38. Wozniak AJ, Crowley JJ, Balcerzak SP, Weiss GR, Spiridonidis CH, Baker LH et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non-small-cell lung cancer: a Southwest Oncology Group study. J.Clin.Oncol. 1998;16:2459-65.
39. The Elderly Lung Cancer Vinorelbine Italian Study Group (ELVIS). Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J.Natl.Cancer Inst. 1999;91:66-72.
40. Comella P, Frasci G, Panza N, Manzione L, Lorusso V, Di Rienzo G et al. Cisplatin, gemcitabine, and vinorelbine combination therapy in advanced non-small-cell lung cancer: a phase II randomized study of the Southern Italy Cooperative Oncology Group. J.Clin.Oncol. 1999;17:1526-34.
41. Comella P, Frasci G, Panza N, Manzione L, De Cataldis G, Cioffi R et al. Randomized trial comparing cisplatin, gemcitabine, and vinorelbine with either cisplatin and gemcitabine or cisplatin and vinorelbine in advanced non-small- cell lung cancer: interim analysis of a phase III trial of the Southern Italy CooperativeOncology Group. J.Clin.Oncol. 2000;18:1451-7.
42. Scagliotti GV, De Marinis F, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in non-small cell lung cancer. ASCO 2001: proceedings of the 37th Annual meeting of the American Society of Clinical Oncology.
43. Schiller JH, Harrington D, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346(2): 92-98.
44. Rubio-Terres et al. An unpublished modeled evaluation of gemcitabine/cisplatin compared with paclitaxel/carboplatin and vinorelbin/cisplatin. Eli Lilly on file, 2001.
45. Nuijten MJC. Data management in modeling studies: the selection of data sources. Pharmacoeconomics 1998; 3: 305-16.
46. Weinstein MC, O´Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health care evaluation: report of the ISPOR Task Force on Good Research Practices - Modeling Studies. Value in Health 2003; 6(1): 9-17.
47. Orlewska E, Mierzejewski P. Polskie wytyczne przeprowadzania badań farmakoekonomicznych (projekt). Farmakoekonomika 2000; Suplement 1: 2-11: http://www.decyzjemedyczne/org.pl [Accessed 2002, April 20].
48. Australian Department of Health and Aged Care. Guidelines for the Pharmaceutical Industry on Preparation of Submissions to the Pharmaceutical benefits Advisory Committee: including major submissions involving economic analyses [online]. Available form URL: http://www.health.gov.au/pgs/pubs/pharmpac/gusubpac.htm [Accessed 2002, Mar 28].

