© Borgis - New Medicine 2/2006, s. 43-47
Joanna Myszkowska-Ryciak, Janusz Keller, Jacek Bujko
The influence of feeding frequency on dietary protein utilization – a model study
Faculty of Human Nutrition and Consumer Sciences Department of Dietetics Warsaw Agricultural University, Warsaw, Poland
Head of Department: Prof. Joanna Gromadzka-Ostrowska, MD, PhD
Summary
Summary
Aim: The purpose of the study was to examine the influence of feeding frequency on dietary protein utilization during growth.
Material and method: A model study was performed on 36 growing, female Wistar rats. Animals were randomly divided into 2 groups (A, B) and maintained in individual cages in controlled conditions with free access to water. After acclimatization each rat from group A was paired with corresponding animal from group B (weight criterion). In pairs animals were fed with the same amount of commercial rats´ diet divided into equal meals: 2 (rat A) or 4 (rat B). Twenty-four hours urine collections were performed twice for each pair of rats: on day 11 and 22; body weight was controlled every day. Starting from day before urine collection all animals received 15N glycine (0.5 mg/g feed) in each meal. After 25 days rats were killed and organs: liver, heart, kidney, small intestine, muscles: gastrocnemius and soleus were excised. Dietary protein utilization was characterized by urine total nitrogen (urea nitrogen and 15N) excretion. Additionally body weight, body chemical composition and organs weight were analyzed.
Results: Statistical analyzes showed no differences in body weight, body chemical composition and organs weight between animals fed 2 and 4 meals a day. There were no differences in urine parameters (total nitrogen, urea nitrogen and 15N).
Conclusions: Feeding frequency of 2 meals or 4 meals a day with the same amount of daily protein during 25 days has no effect on dietary protein utilization in growing female rats.
Introduction
In the papers concerning the rules for proper nutrition we can find the recommendation for eating three meals a day. While treating obesity, feeding older persons and children, eating 4 to 5 meals a day seems to be more advantageous. Leciejewska [1] showed the existence of significantly higher thermogenic response and as a result worse energy utilization in people having two smaller meals a day as compared with one bigger, whereas Bellise et al. suggest [2] that there are no significant differences in daily energetic consumption while having meals at high or low frequency. In spite of this, there is a theoretical possibility that digesting and absorption of nutritional components consumed in the form of smaller but more frequent meals can affect the metabolic routes of their conversion in the post-meal period. Studies performed on laboratory animals showed, among others, a high rate of glycogen synthesis and lipogenesis de novo occurring after one big meal, whereas it did not occur following a few smaller meals [3]. At the same time the possibility of improving the use of protein achieved by consuming a larger number of meals at a constant protein level in meals support further results of studies on animals, performed among others by Batterham and Bayley [4], Weijs et al. [5], Schiffelers et al. [6] and Bujko et al. [7]. On the other hand, the studies performed by El-Khoury et al. [8] did not confirm any significantly advantageous impact of meal frequency on the utilization of proteins in humans.
The aim of this paper was to assess the interdependence between the dynamics of protein consumption and its utilization in the model experiments performed on laboratory rats of Wistar breed. This paper includes a hypothesis that the utilization of food proteins consumed during a day is influenced by the number and amount of meals.
Material and method
The experiment was performed on 36 young (5-6 week old) growing rat females of Wistar breed with mean body weight of 94 ± 6.8 g coming from the breed of Physiology and Nutrition Institute of Polish Academy of Science in Jabłonna near Warsaw. The animals were kept in animal quarters of the Faculty of Consumption and Human Feeding, SGGW (Warsaw Agricultural Academy) in single metal cages, in rooms under controlled conditions (temperature 22-23°C, air humidity 50-60%, daily light cycle 12/12 h) with permanent access to water. At the dark stage (8.00-20.00) of the daily cycle red light was used, enabling to perform necessary operations i.e. feeding and weighing the animals. The experiments included a standard diet: Labofeed B for rats (protein – 17.0%, energy – 12.15 MJ/kg) produced by the Feed Plant of Andrzej Morawski, ground before application in a metal (mill) grinder, then the feed was mixed with water at the proportion of 1:1. During the three days of initial stage aiming at getting animals used to the experimental feeding schedule, randomly selected half of animals (n=18) was fed ad libitum with two meals a day (group A; 9.00-10.00, 18.00-19.00). Remaining animals (group B; 9.00-9.30, 12.00-12.30, 15.00-15.30, 18.00-18.30) were fed ad libitum with 4 meals a day.
In the experimental period lasting 22 days for the first four days the feed consumption was monitored during the first meal, then mean (average) daily feed consumption was calculated for each animal. After this period (day 5) each rat from group A was assigned an animal from group B matching them by body weight. Animals in pairs (n=18) were given the same amount of feed divided into two meals (animals A) or four (animals B) animals. The dose of feed was defined based on mean daily consumption of a rat from group A, receiving feed two times a day. The body weight of those animals was checked everyday before the first feeding. During the first proper period the amount of feed every 4 days for each pair of animals was increased proportionally to body weight of the rat that received 2 meals a day (per 10% of growth the feed amount was increased by 8%). The feeding procedure was continued for next 5 days, then the animals on day 10, preceding the first 24-hour urine collection in metabolic cages were given glycine marked with isotope N15 (Sigma Aldrich Chemical Company, St. Louis, USA) at the amount of 0.5 mg/gram of feed in each meal. Application of marked glycine was continued till the next 24-hour urine collection (on day 22). The urine collection was carried out in metabolic cages made of plexi-glass (Techniplast, Italy, 320 cm3) every 10 days. The collected urine after acidification with 10% sulphuric acid solution [9] was frozen at the temperature of -22°C and stored for further chemical analyses. After 25 days of experiments animals were put to sleep to collect organs (heart, liver, kidneys, small intestine, soleus and gastrocnemius muscle), which after rinsing in physiologic salt solution and drying were weighed at accuracy of 0.0001 g. The rest of animal carcasses were frozen at the temperature of –22°C and stored for further analyses.
The total nitrogen content was determined by using Kjeldahl method with automatic analyzer type Kjeltec Auto 1002 ANALYZER manufactured by Foss Tecator company [10]. Raw fat content was determined by using Soxhlet method. Dry matter weight and ash weight was determined with the method described in Official methods of analysis of The Association of Official Agricultural Chemists [11] in the laboratories of the Section of Dietetics and Functional Food, Dietetics Department of Warsaw Agricultural University (SGGW), Warsaw. The analysis of 15N content in rat urine was performed by using mass spectrophotometer ANCA GSL manufactured by an English company Europa PDZ in the Department of Dietetics, SGGW. Urea concentration in urine was determined by using a COBAS Integra appliance in the Central Laboratory of the Independent Central Public University Hospital In Warsaw with a set of reagents by Roche Diagnostics.
The studies granted permission from the III Local Ethics Commission at SGGW.
The results were analyzed by using the T student test for independent data (coupled in pairs) [12].
Results
No statistically significant differences were observed at the body weight growth in rats receiving the same amount of food in two or four meals The average daily (24-hour) body growth amounted to 2.0 g/24h in animals receiving 2 meals a day, whereas 2.1 g/24h in rats receiving 4 meals. Statistical analysis did not show any significant differences in final body weight between animals receiving feed in two meals for 25 days (144±16.7 g) or in 4 meals (146±18.0 g). The average feed consumption per 100g of body weight amounted to 13.4 g/24h in animals fed twice a day and 13.2 g/24h in rats receiving 4 meals.
Data concerning the composition of body presents Table 1. The chemical composition of body was determined in animal carcasses obtained after removing examined internal organs (heart, liver, kidneys, small intestine, soleus and gastrocnemius muscle), the results were expressed in the percentage of fresh weight. The weight of those bodies was by about 8% lower than animal weight determined intra vitam.
Table 1. Chemical composition of rats´ carcasses.
| Group | Dry mater [%] | Protein [%] | Fat [%] | Ash [%] |
| A (2 meals) | 32.9?1.41 | 18.1?1.80 | 8.5?1.88 | 3.83?0.11 |
| B (4 meals) | 32.5?1.74 | 17.9?1.78 | 8.0?2.13 | 3.92?0.06 |
Statistical analysis did not show any significant differences in chemical composition of rat bodies being assessed: between those having two or four meals a day.
The weight of collected organs (heart, liver, kidneys, small intestine, soleus and gastrocnemius muscle) expressed in percents has been presented in Table 2. Statistical analysis did not show any significant differences in the rat body weight between the ones receiving two or four meals a day.
Table 2. Organs´ weight expressed as percentage of body weight in groups fed with 2 and 4 meals a day.
| Group | Liver [%] | Kidney [%] | Heart [%] | Small intestine [%] | M. soleus [%] | M. gastrocnemius [%] |
| A (2 meals) | 3.58?0.32 | 0.85?0.05 | 0.33?0.03 | 2.98?0.44 | 0.05?0.00 | 0.12?0.02 |
| B (4 meals) | 3.68?0.34 | 0.85?0.05 | 0.33?0.03 | 3.14?0.45 | 0.04?0.01 | 0.10?0.01 |
Statistical analysis did not show any significant differences in the excretion level of total nitrogen (Figure 1), urea nitrogen (Figure 2) and nitrogen N15 (Figure 3) with urine between animals receiving a daily portion of feed in 2 or 4 meals. No significant impact of 10-day period of experimental feeding schedule on examined parameters has been found.

Fig. 1. Average nitrogen contents in rats´ urine on day 11 and 22 (mg/day/100 g of body weight).
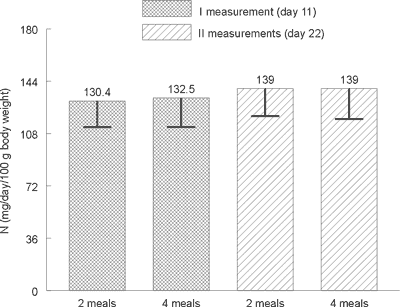
Fig. 2. Average urea nitrogen contents in rats´ urine on day 11 and 22 (mg/day/100 g of body weight).
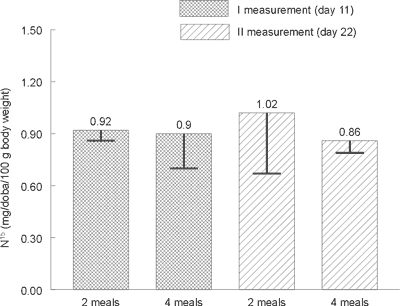
Fig. 3. Average isotope 15N nitrogen contents in rats´ urine on day 11 and 22 (mg/day/100 g of body weight).
Discussion
Gevers et al. [13] found that that body weight growth rate in rats depends mainly on the frequency of growth hormone (GH) release. The release of GH in rats shows two peaks – at the beginning and at the end of activity in the dark stage – which suggests the connection of GH release with periods of increased food intake [14, 15]. Vaccarino et al. [16] suggest that it can improve the utilization of diet composition, including contained protein, consumed during those two specific periods. In this case rats receiving the daily feed portion in two meals: at the beginning and at the end of the active stage can feature bigger body mass growth or bigger growth of total protein weight as compared with the animals having 4 meals. At the same time scientists found that rats having access to feed for a longer period, cannot eat as much feed characterized by proper nutritional components, which results in lower body mass as compared with control groups fed ad libitum. A large amount of protein consumed in one meal cannot exceed the body ability to synthesize this element, thus cannot increase its oxidation. On the other hand many authors showed that animals having only one meal create behavioral, biochemical and physiological adaptive mechanisms leading to metabolic changes improving the use of nutritional components (mainly by increase in post-meal ”storing”). These changes consist in increasing intestinal absorption [17], increasing the glycogen synthesis in muscles and liver [18], increasing lipogenesis in fat tissue [19], reducing the metabolism at rest [20] and reducing physical activity [21]. In addition, Arnal et al. [22] found that the increase in post-meal protein synthesis in liver and gastrocnemius muscle in rats receiving a daily dose of feed in one big meal (66% of daily protein demand) and three small ones (3 x 11% of daily protein demand) as compared with the group where identical amount of food was divided into 4 equal meals (4 x 25% of daily protein demand). Tessari et al. [23] showed that in the case of dividing the food with the protein content of 0.8 g/kg of body mass, into smaller, isoenergetic meals given every 20 minutes within 4 hours, protein synthesis has grown by 30%. It was shown not only by increasing the protein synthesis rate but also by the drop in proteolysis in muscles, whereas Weijs [24] suggests that the occurrence of higher protein retention in the post-absorption period in rats fed with 6 smaller meals as compared with 2 larger meals at marginal way of feeding results in better protein status and bigger body weight growth in animals.
In the performed experiment no differences were found both in body weight growth and in protein body mass of the animals after 25 days of applying meals at a differentiated frequency. In addition, no differences were found in the weight of internal organs. These results comply with the results obtained by Arnal et al. [22], who in spite of some differences in post-meal protein utilization depending on the feeding way did not observe any significant differences in body mass, in the weight and nitrogen content in gastrocnemius muscle after 21 days of experiment. It suggests that meal frequency has no impact on the long-term utilization of protein measured by the above-mentioned parameters. Based on the literature-obtained data we can presume that the applied feeding schedule could have an impact on post-meal utilization of this component that cannot be measured with traditional balance or growth methods. As the additional utilization marker for food protein we assumed the level of total nitrogen expelled with urine, urea nitrogen and additionally isotope 15N coming from the glycine marked with isotopes.
The increase in protein consumption results in reducing the synthesis of urea and excretion of this substance by kidneys; although its excretion level never achieves zero level because there is always a minimum amount of amino acids being subject to degradation even at diets completely free from proteins. The natural and necessary degradation of amino acids is connected with constant exchange of intracellular and blood plasma proteins. In addition, under the conditions of normally applied feeding the process of gluconeogenesis occurs (especially at night), which results in increased degradation of amino acids. The fact that the level of expelled urea is lower that its synthesis is also of importance. Urea is subject to hydrolysis with micro flora in bowels along with releasing ammonia [25]. While searching literature one can find the statement that the regulation of urea synthesis has an impact on the systemic nitrogen balance control [26].
The dependence of general urea excretion on the level/way of feeding has been a subject of many studies. For example Young et al. [26] showed a linear correlation between consumption of protein/nitrogen and the production and excretion of urea. The studies of Daenzer et al. [27] suggest that the level of total nitrogen expelled in urine depends not only on the protein content in food but also the administration method. They showed a significantly higher amount of nitrogen within 24 hours in rats fed with food containing free amino acids as compared with animals receiving identical amount of energy and nitrogen in the form of protein (casein), which indicates higher protein retention in rodents being on a protein-containing diet as compared with those fed with food containing free amino acids. The performed experiment did not show any differences in the excretion of total nitrogen, urea nitrogen, as well as isotope 15N coming from the marked glycine when daily feed portion is divided into different number of doses (proteins). The determined nitrogen content in urine reflects the total daily protein consumption, whose chemical form and amount was identical, whereas the differences referred only to consumption time.
Conclusions
In the model study performed the increase in number of meals from 2 to 4 at maintaining a constant feed dose (protein) and using this kind of feeding for 25 days in growing rat females does not show a significant impact on dietary protein utilization measured by excretion of nitrogen and body weight growth.
*KBN grant no 3 P06T 050 245 P0.
Piśmiennictwo
1. Leciejewska A. Dietary-induced thermogenesis in men as influenced by meal frequency and composition. 15th Symposium on Energy Metabolism in Animals, Denmark, 2000. 2.Bellise F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr 1997; 77: 57-70. 3.Fabry P, Tepperman J. Meal frequency: a possible factor in human pathology. Am J Clin Nutr 1967; 20: 816-24. 4.Batterham ES, Bayley HS. Effect of frequency of feeding of diets containing free or bound lysine on the oxidation of 14C lysine or 14C phenyloalanine by growing pigs. Br J Nutr 1989; 62: 647-55. 5.Weijs PJM, Schreurs VVAM, Maas MIM. Meal frequency and diurnal leucine oxidation in mature rats. J Animal Physiol Animal Nutr 1995; 74: 131-6. 6.Schiffelers SLH, Schreurs VVAM, Krawielitzki K, Koopmanschap RE. Effect of meal size on protein metabolism and utilization of dietary amino acids at a marginal level of protein intake. 7th International Symposium on Protein Metabolism and Nutrition, EAAP publication 81: 345-7, 1996. 7.Bujko J, Schreurs VVAM, Koopmanschap RE, Furstenberg E, Keller JS. Benefit of more but smaller meals at a fixed daily protein intake. Z Ernahrungswiss 1997; 36: 347-9. 8.El-Khoury AE, Sanchez M, Fukagawa NK, Gleason RE, Tsay RH, Young VR. The 24-h kinetics of leucine oxidation in healthy adults receiving a generous leucine intake via three discrete meals. Am J Clin Nutr 1995; 62: 579-90. 9.Tomaszewski J. Diagnostyka laboratoryjna dla studentów medycyny. Warszawa, PZWL, 1997. 10.Persson JA. Poradnik mineralizacji Kjeldahla. Przegląd metody klasycznej z ulepszeniami dokonanymi przez firmę Foss Tecator. Foss Tecator, AB, 1966. 11.Horwitz W, Robertson AH, Fisher HJ, Epps EA. Official methods of analysis of The Association of Official Agricultural Chemists, Washington, AOAC 9th ed, 1960. 12.Stupnicki R. Biometria. Krótki zarys. Warszawa, Wyd Margos, 2000. 13.Gevers EF, Wit JM, Robinson IC. Growth, growth hormone (GH)-binding protein, and GH receptors are differentially regulated by peak and trough components of the GH secretory pattern in the rat. Endocrinology 1996; 137(3): 1013-8. 14.Strubbe JH, Keyser J, Dijkstra T, Prins AJ. Interaction between circadian and caloric control of feeding behavior in the rat. Physiol Behav 1986; 36(3): 489-93. 15.Strubbe JH, Spiteri NJ, Alingh Prins AJ. Effect of skeleton photoperiod and food availability on the circadian pattern of feeding and drinking in rats. Physiol Behav 1986; 36(4): 647-51. 16.Vaccarino FJ; Sovran P, Baird JP, Ralph MR. Growth hormone-releasing hormone mediates feeding-specific feedback to the suprachiasmatic circadian clock. Peptides 1995; 16(4): 595-8. 17.Leveille GA, Chakrabatry K. Absorption and utilization of glucose by meal-fed and nibbling rats. J Nutr 1968; 96: 69-75. 18. Fuller R, Diller ER. Diurnal variation of liver glycogen and plasma free fatty acids rats fed ad libitum or single daily meal. Metabolism 1970; 19: 226-9. 19.Hollifield G, Parson RW. Metabolic adaptations to a ´stuff and starve´ feeding program. Studies of adipose tissue and liver glycogen in rats limitted to a short daily feeding period. J Clin Invest 1962; 41: 245-9. 20.Hill JO, Latiff A, DiGirolamo M. Effects of variable caloric restriction on utilization of ingested energy in rats. Am J Physiol 1985; 248: 549-59. 21.Leveille GA, O´Hea EK. Influence of periodicity of eating on energy metabolism in the rat. J Nutr 1967; 93: 541-5. 22.Arnal MA, Mosoni L, Dardevet D, Ribeyre MC, Bayle G, Prugnaud J, Mirand PP. Pulse protein feeding pattern restores stimulation of muscle protein synthesis during the feeding period in old rats. J Nutr 2002; 132: 1002-8. 23.Tessari P, Barazzoni R, Zanetti M, Vettore M, Normand S, Bruttomesso D, Beaufrere B Protein degradation and synthesis measured with multiple amino acid tracers in vivo. Am J Physiol 1996; 271: 733-41. 24.Weijs PJM. Dietary Protein, Physiological Condition and metabolic amino acids utilization. Ph.D. Thesis, Department of Human and Animal Physiology, Wageningen Agricultural University, Wageningen, The Netherlands, 1993. 25.Walser M, Bodenlos LJ. Urea metabolism in man. J Clin Invest 1959; 38: 1617-26. 26.Young VR, El-Khoury AE, Raguso CA, Forslund AH, Hambraeus L. Rates of urea production and hydrolysis and leucine oxidation change lineary over widely varying protein intakes in healthy adults. J Nutr 2000; 130: 761-6. 27.Daenzer M, Petzke KJ, Bequette BJ, Metges CC. Whole-body nitrogen and splanchnic amino acids metabolism differ in rats fed mixed diets containing casein or its corresponding amino acids mixture. J Nutr 2001; 131: 1965-72.


