© Borgis - New Medicine 4/2011, s. 130-137
*Halina Osińska1, *Leo Barić2
Personalized medicine. Vision and Reality
1Chairman of the Board of Polish Society of Health Education
2Consultant, Retired Professor University of Manchester and University of Salford
Summary
The decoding of the DNA sequence and interpreting the causal association between the genetic profile and health threats, sensitivities and future disease, represents a break in the traditional understanding of the health issues and of preventive and curative approaches. It is a paradigm shift which will take us into new areas of knowledge, understanding and interpretations concerning health matters. It is obvious that it will have considerable effect on the way we approach treatment of a holistic patient with a health problem and not just a health problem.
The article addresses the issue of the macro and micro approach to the introduction of personalized medicine and illustrates some benefits of each. It also makes suggestions about how to provide resources for financing this change and makes a case for the savings to come from returning the management of health care as a servicing industry into the hands of the doctors and nurses who know what it is all about to meet the needs of a patient
The initiative in Faroe Islands based on genetic screening of the whole population as the precondition for the introduction of ‘personalized medicine’ will provide the basis for implementation of this new approach. The case study in Warsaw, providing the medical staff with additional social skills to fulfil their new role will provide a tested curriculum as a complementary aspect of the introduction of personalized medicine.
PROLOGUE
On the 30.9.2011 Mark Henderson, Times Science Editor, wrote:
Islanders lead world with DNA test for entire population
The Faroe Islands will become the first country in the World to read the entire DNA code of every willing citizen in an initiative that could lead to a new era of personalized medicine internationally.
All 50.000 of the islands inhabitants are to be invites to have their genomes sequenced and linked to their medical records, to tailor health care to individuals’ DNA and build a resource for research.
The ambitious FarGen project will examine how doctors can use individual patients’ DNA to select the best therapy and to predict and reduce their risk of developing certain diseases.
It will also explore the social challenges of mass genome sequencing. These include the logistics of collecting, storing and interpreting DNA data from a whole population, the consequences for privacy and insurance, the ethics of sequencing children, and confidential access for medical research.
Bogi Eliasen, the program manager of the Faroes’ Department of health is in charge and he said that Faroes’ small population make it ideal to pioneer comprehensive genomic health care and study its social implications. The movement is accompanied by school lectures on genomics so that people would understand what it means, its benefits and its risks.
INTRODUCTION
From the above article it seems that the introduction of ‘personalized medicine’ is taking place. It has two aspects, the changes in the provision of services and the professional treatment of the patients. The isolated population of Faroe Islands will serve as an excellent research material for testing the implications of the introduction of ‘personalized medicine’ into the health care system and will indicate the additional knowledge and skills needed for the medical personnel to meet the new demands. These demands are the subject of the case study in progress at present in Poland, which is studying the introduction of embedded HE/HP, as a way to provide this additional knowledge and skills to the health care professionals to meet the patients’ demands.
To meet these challenges and to carry out a prospective impact assessment of the implementation of the new ‘Barić/Osinska Model 2010’ of embedded HE/HP, an initiative is taking place in Poland, where the Polish Society for Health Promotion is sponsoring a research project to be carried out at the Hospital Brudnowski of the Warsaw Medical University in the Department of Internal Disease and Diabetes, under the Director Professor Dr Jan Taton. The embedded health education and health promotion (HE/HP) represented as ‘Barić/Osinska Model 2010’ is aimed to meet these new demands and represents a new approach for the medical profession to help people improve health, manage disease and cope with their consequences within the new ‘personalized medicine’ (1).
In this way, the present studies will cover the both aspects of the introduction of personalized medicine, which are the structural/functional adjustment of the health care system and the additional knowledge and skills of the health personnel (2, 3).
Before we describe the two aspects of this adjustment it will be necessary to clarify this new concept (see diagram):
It is true that on the patient level, medicine has always been personal in the form of doctor/nurse-patient interaction. The new concept of personalized medicine is, however, reflecting the paradigm shift due to the decoding of the human genome, with all its consequences for prevention and treatment of disease. The new concept of ‘personalized medicine’ means a new tailor made treatment of an individual including the new approaches to establishing the way the treatment is planned and carried out. It also means a change in the prevention by introducing genetic screening as a way of defining personal as compared to the population risk. All this will influence the interaction between the doctor/nurse and the patient. This is a direct consequence of the new developments in genetics. After the initial decoding of the human genome, the understanding of its meaning for humans and their health has been rapidly developing. One of these developments within preventive medicine, relevant to HE/HP has been genetic screening. This aspect of genetics has made great advancements and at present there are a number of tests available for different diseases and/or health threats as well as for other purposes such as in crime detection, in assessing parenthood, in assessing risk from certain future health threats and diseases etc. The screening process provides information about genetic associations with certain disease as well as certain risk factors leading to a potential disease or genetic condition (3).
In summary, the introduction of personalized medicine will require the genetic screening of patients by the health care institutions for the purpose of providing “tailor-made” treatment and information about potential risks from diseases for individuals, as well as providing the health care personnel with additional knowledge and skills to meet these new patients’ demands.
PERSONALIZED MEDICINE
Introduction (4, 5)
The genetic profiling based on sequencing individual DNA is starting to be accepted as the basis for a personalized health care. This is especially true for curative medicine, which in the past depended on medicines which used epidemiological methods to study the distribution of a health threat in a population, find out appropriate medicines by laboratory research, animal studies and eventually selected population studies for a disease (say cancer) and in the hope that the wide application of such a treatment on a cancer population will show a certain improvement for at least some of the patients. Such population studies also raised the question of predicting outcomes on a personal level. Current health care approach has been extremely expensive (using 10-17% of GDP in western countries) and very inefficient, where the Adverse Drug Reactions constitute the 4th leading cause of death because of population based studies which produced “one size fits all” treatments.
The curative aspect of personalized medicine is based on targeted therapy for each individual patient. This will be possible if the research aims to develop drugs appropriate for specific molecular and genetic targets in subsets of patients with similar genetic profile. These specific drugs will have a dramatic effect on such subsets of patients compared with more generalized drugs which produce only minimal improvement in a larger population group.
A great step in the introduction of personalized medicine has been described by Mark Henderson, science editor of The Times (9.11.2011) with the introduction of a genetic test known as Snapshot at Massachusetts General Hospital (MGH) in Boston which has been shown to be sufficiently fast and accurate to guide treatment choices in lung cancer. The procedure analyses tumours for genetic defects to help doctors to select most effective treatment. It looks for more than 50 mutations in 14 cancer genes. A similar test is to be introduced to Britain on a pilot basis by charity Cancer Research UK. The latest genetic research findings however show not only that several genes can be associated with one disease, but that one gene can be responsible for a number of diseases (The Times, 12.11.2011). The study by the University of Edinburgh found that genes responsible for Crohn’s disease are linked with other conditions such as breast and prostate cancer, Hodgkin’s lymphoma, high cholesterol and obesity. The same has been found for people carrying particular genes responsible for heart disease, Parkinson’s disease and some cancers who could be at risk from developing other health problems. This is an important insight into the genetic background of developing medicines and shows the complexity of this newly discovered area of medicine (6).
Knowing a DNA profile may greatly improve the effectiveness of treatment and response to drugs for each individual patient. Since, however such treatments can be very expensive, the ethical question of prescribing will be accompanied by economic and political decisions.
Within preventive medicine the establishment of links between genes and disease has had an uneven development and in some areas (according to the popularity of the health threat such as cancer) it has been rapid whereas in others the pace is more modest. Scientists have discovered hundreds of genetic variants associated with different diseases. This has been accompanied by rapid reduction in the cost of tests from 2 billion for sequencing the human genome to ?1000 for a personal DNA profile within the next 5 years, whereas a partial genetic test as for proving paternity is being advertised for ?100. Most recently, however (Henderson in The Times 15.10.11 writes) that the Newcastle-based company QuantuMDx has already built a prototype of a hand-held device that can read a patient’s DNA in 20 minutes for as little as ?10 per test, which is being tested in Africa for HIV before it is ready for the Western health care systems. This will make it affordable not only for hospitals but also for general practices and pharmacies.
These developments have had a considerable impact on the ways the prevention of disease is being carried out. One example is the prevention of breast cancer. Years ago the way to prevent the negative effects of the disease, although not to prevent cancer, has been the early discovery of first signs of the disease. The available method was ‘breast self-examination’, where the women regularly examined their breasts, and when discovering a ‘lump’ were supposed to seek medical help. The method was not very efficient because of a high level of false positives, resulting in unnecessary invasive treatment. The great improvement of this idea of early detection was the introduction of mammography or breast examination by professionals to discover early abnormalities. Wide spread application of the test resulted in the discovery of increased number of abnormalities only some of which have been malignant with still a high number of false positives. At present in UK an official inquiry into this method is in progress because of high numbers of false positives and unnecessary mutilations of women. At the same time a number of elderly groups of women are being offered genetic screening, which should increase the percentage of malignant growths as well as indicate the best way of their individual treatment.
Another example is the improvement in genetic testing, such as in the detection of Dawn’s babies. The present invasive tests for Dawn’s can cause miscarriage (in 1/100 or 1/200 cases) and consisting of amniocentesis or chronic villius sampling where a needle is inserted into the womb to collect amniotic fluid or a piece of placenta for genetic testing. The new non-invasive pre-natal diagnosis (NIPD) is a blood test which isolates high risk groups and reduces risk of miscarriage and is now being offered in US and will soon be offered in UK. It consists of giving pregnant women a “nuchal translucency test” which measures the thickness of foetus’s neck, which is thicker in Dawn’s babies. It is performed between 11 and 14 weeks and the results are analysed using mother’s age and possibly blood tests. At-risk women are then offered the NIPD to look at DNA from the foetus, which if having three copies of chromosome 21 instead of two indicates positive risk. The selected group of high risk women (because of low levels of false positives) will be offered further testing using chronic villus sampling before 15 weeks or amniocentesis if later, especially if they are considering an abortion. In this way the Dawn’s risk is confirmed usually in 14-17 weeks and gives parents more time to decide on the next step or prepare to live with a Dawn’ baby.
One can assume that in not so distant future most of people having access to a developed health care system will have access to genetic screening and will make use of it. This may happen because a person is curious or prudent in managing his/hers health problems or as a part of regular medical treatment. It could also be the result of coercion associated with job applications, insurance policies or other social pressures. If such screening occurs at birth, the medical profession could be faced, for example, not only with the problem that the child is the carrier of a genetic mutation associated with cystic fibrosis but also with a mutation which is associated with the risk of addiction to alcohol as compared to habitual social drinking. In this way the medical profession will not only have to deal with potential disease but also with potential behavioural risks for each person.
This raises the problems for medical education, which not only will require inclusion of a greater knowledge of genetics and all its consequences for health and health care delivery, but also embedded HE/HP knowledge and skills as one way for helping patients to cope with this increase in information and its consequences. This issue is dealt with in the next section of this paper (6-21).
The implementation of personalized medicine (22, 23)
The introduction of personalized medicine, represents a big step in social innovation, and will require a sensitive approach based on prospective impact assessment of each phase and each area of change. This process can be divided into three phases, the macro-micro approach, the issue of financing the change, and on the patient level the training of the personnel to meet the new demands (see diagram):
Phase 1: Macro and micro changes (24)
The introduction of personalized medicine can be carried out on two levels (see diagram):
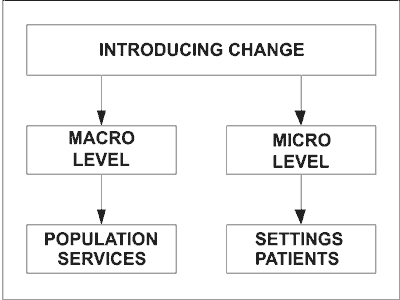
The introduction of changes on a macro level includes changes in the health care services and a population approach to prevention. The former includes the provision of genetic testing in various health care settings and the latter includes the screening for specific population groups. The best example is the study carried out on the entire population of the Faroe Islands. Another example is the planned pre-conceptual screening of young people over 16 in Great Britain and testing of Scottish families with over 4 children to establish the connection between parental age and DNA mutations. There is also at present on offer genetic screening for breast cancer. Most recently an un-named premier league football club has had a DNA test carried out on all its players to discover who is susceptible to rupture of tendons, a common footballing injury (writes Beezy Marsh in Sunday Times,16.10.11).
In all those cases, the planned screening will provide a data base of relevant information, which will demand the adjustments in each and every health care setting, where the relevant information can be used.
This raises a whole set of ethical problems, of who owns the data, who controls the data, for what purpose the data will be used and what protection measures will be put in place to protect people who’s DNA becomes public property.
In Faroe Islands this approach will require simultaneous adjustment of the whole health care system to meet the new demands as well as additional training of health personnel to meet these new demands.
The pre-conceptual screening in UK will place a great demand on primary health system to deal with a whole population faced with new information and needing advice, coaching and counselling to cope with it. It will also place a demand on social services and the legal system due to all kind of psycho-social problems and litigation in addition to medical ones.
The introduction of personalized medicine on a micro level means introducing change on the settings level. It will require the health care settings, such as hospitals, to establish genetic screening clinics for their patients. This will enable the hospital personnel to use the genetic data to adjust the treatment to individual patient’s needs.
It will also mean the introduction of change in the extent of a differential diagnosis, which will now for each patient include not only medical but also psycho-social parameters as the result of the genetic profile of that patient.
This approach allows enough time for each setting to adjust to needs and prepare the staff for change in demands. It would also allow for prospective impact assessment, where the staff of the setting could be the experts within a Delphi approach to express their opinions about the proposed changes.
The problem facing the decision makers is, which approach is less threatening and more likely to succeed. The existing experience shows that a macro approach involving a population is necessary to change norms in a society, whereas a micro approach has the advantage of staff participation in the introduction of change and thus increasing the probability of a lasting success.
Phase 2: Financing the changes
There is no doubt that introducing such a radical change in a whole health care system will require considerable resources. The simplest way is on a macro level to obtain these resources from the stake holders of the health care system. This can be Government, insurance companies or private sources according to the way the health care in that country is financed.
There is, however on a micro level, another way to obtain resources necessary for financing change, which comes from the savings made by each setting. The easiest way of securing these savings is from restructuring the setting by returning the management of the setting into the hands of the medical personnel. At present there is no empirical evidence to show that the introduction of lay managers into hospitals has improved the health outcome of the patients.
The present lay managers came into health care service industry from manufacturing and brought their models and approaches with them, as for example:
– They introduced models from manufacturing industry into the service industry;
– They turned service settings into marketing settings and patients into consumers;
– They introduced selling and buying services as commodities;
– They established the profit/loss concept in a budget based industry;
– They introduced targets and disregarded the care element;
– They introduced competition between settings based on cost saving indicators;
– They introduced quality assessment based on management and not medical parameters (etc.).
In the days of patient oriented and evidence based services, the lay managers nearly doubled the cost of the services without any evidence that the quality of health care to the patients doubled due to their contributions.
The most telling argument, however is, that the lay managers manage a setting without knowing what goes on in that setting between the doctor/nurse and the patient, or what should go on. The original idea was ‘let doctors do doctoring and managers managing’ which was never tested by prospective impact assessment and now has backfired, and we have such anomalies as Government financed hospitals going bankrupt, or closing wards in non-profitable hospitals and sacking medical personnel to save the cost.
The halving of the cost of a setting would provide enough resources for the establishment of a genetic screening unit and training the personnel in new approaches in dealing with new demands of the patients.
Phase 3: The patient level
On the patient level, personalized medicine will require that the doctors and nurses obtain additional knowledge and skills to meet the new and additional demands of patients. This issue is being addressed in a separate section of this paper.
TRAINING THE MEDICAL PERSONNEL
There has been in the past very little evidence that a patient, when faced with a doctor or nurse in a hospital situation, would be inclined or motivated to initiate a discussion of some personal problems in addition to the main health problem that caused hospitalization. With the availability of genetic profiling and the possibility of establishing the genetic predispositions or links to some present or future disease, the situation has radically changed. Both doctors/nurses and patients may be more than willing to discuss in addition to medical some psycho-social issues arising from the comprehensive differential diagnosis as a result of ‘personalized medicine’.
It will be for the health professional, when conducting such a differential diagnosis of the patient, to examine in addition to medical aspect, also any psycho-social aspects which may be relevant for the treatment and management of the condition presented. This differential diagnosis as a part of personalized medicine will include medical as well as any relevant psycho-social parameters. When discussing the findings with the patient, the doctor/nurse will provide information about all relevant aspects and negotiate with the patient the planned medical as well as psycho-social treatment (25).
The embedded HE/HP approach
Faced with additional aspects of treatment of a patient, including some insights from the screening results concerning present and future sensitivities or direct causes of certain diseases, affecting the patient directly or his/her children, the doctors and nurses will need to confront and deal with this challenge. For this they will need the additional knowledge and skills offered by embedded health education and health promotion (HE/HP), as described in the ‘Barić/Osinska Model 2010’ (26).
The main postulates of embedded HE/HP can be summarized as follows:
– The personalized medicine is the result of the recent developments in genetics and by screening offers the possibility to establish individual sensitivities requiring tailor made individual treatment; not all of these insights are of a medical nature and some will have strong psycho-social characteristics requiring HE/HP methods of interventions;
– We are talking about a hospital where doctors and nurses are practicing ‘personalized medicine’ in treating and caring for patients, and for this they need social as well as medical caring skills, which include also HE/HP methods as a part of their core activity;
– The social caring skills within embedded HE/HP should be a part of doctors and nurses core activities and include the methods of informing, coaching and counselling (HE) and lobbying, mediating and advocating (HP); these skills can be acquired either by in-service workshops or as a part of revised medical curriculum;
– The HE/HP aspect of the treatment process is never imposed on the patient (however necessary it may be), but based on the differential diagnosis, it should be a result of the negotiation process between the doctor/nurse and the patient and follow the patient’s approval;
– The extent of the HE/HP aspect of treatment will be dictated by each patient, who should always be in a position of control as to the extent of the exposure and should never be forced to reveal information about their personal life and habits, which they are not willing to do;
– Since the HE/HP aspect of treatment is a part of the core activity it cannot be evaluated as such; the only possible way to evaluate it will be if treated as value-added contribution to the care of the patient; this will be based on special indicators and criteria for each case;
– The doctors and nurses will never know when they will be required to use HE/HP knowledge and skills when dealing with a patient; therefore they will have to be trained in the various methods of HE/HP aspects of their interaction with a patient so that they will be ready when the need arises; this they will acquire through special workshops whereas the new generation of doctors and nurses should get it during their regular studies.
Once an environmental, social and/or psychological aspect of a medical condition has been established during the differential diagnosis, the general assumption is that the doctor/nurse will inform the patient as a part of general medical treatment, and the patient will willingly accept the suggestion and follow the advice (27-30).
This assumption is not supported by any available evidence. On the contrary, doctors and nurses have plenty of evidence about the lack of conformity to recommended medical treatment. Conformity to psycho-social aspects of the prescriptive treatment has not been widely tested and it can be assumed that it will manifest the same patterns as with medical treatment, if not worse (27).
There have been a number of models developed to deal with patient’s motivation, most of which have been applied in health promotion, although some have been developed for health education. The embedded approach being used by doctors/nurses in their interaction with a patient postulates that there are a number of mechanisms, which could motivate a patient to accept and follow the received advice concerning both the medical and psycho-social aspects. These mechanisms have been the subject of a great number of research efforts, resulting in numerous models and theories. For the purpose of the embedded approach for doctors and nurses it will be necessary to simplify the choice and recommend the use of two:
– The research into perception with special reference to patients perception of the advice concerning the psycho-social aspects of their condition as a part of medical advice relevant to treatment and not something separate where the patient has a choice to accept it or not;
– The research into motivation and finding the best way of enhancing patient’s motivation to follow and persist in following the received advice.
The issue of perception
The patient’s perception of advice will depend on the way the doctor/nurse present it and the way the patient will understand, interpret and accept it. The doctor/nurse who have integrated embedded HE/HP into their core activities, when carrying out the collection of information about the history of the disease and when making differential diagnosis, will include some relevant (and not routine or randomly chosen) psycho-social parameters in addition to the relevant medical parameters and present them to the patient (27).
The patient will accept the combined advice as a normal outcome of the differential diagnosis and will (in most cases) conform to the demands of the newly acquired sick role. This advice will be individually tailored to the needs of that particular patient (for example there are dozens of combinations of different factors associated with Diabetes as can be seen in ICD E10-E14, and for each patient the advice will have to meet the demands of that patient’s specific combination). Finding the right combination for each patient will require professional knowledge and skills, which should be acquired during the in-service workshop. When informing the patient of the differential diagnosis and suggesting the course of treatment, the doctor/nurse enters into the process of negotiation ending in acquiring the patient’s agreement to the prescribed treatment. In some cases, such as with surgical interventions, a formal signed consent will be required, whereas in other cases a verbal agreement will be sufficient. This will cover both the medical and psycho-social aspects if that is how they are presented to the patient. When presented in this way, the patient will have no problems in perceiving the relevant psycho-social elements as a part of a comprehensive differential diagnosis (27).
The issue of motivation
The doctors/nurses and the patients have been often chosen as subjects for the study of motivation, which looked at various psycho-social aspects of these actors, their behaviour and their interaction. A number of them have been interested to find out what motivates the various actors to carry out or not various actions. This has resulted in a great number of models and theories on how to motivate their patients to accept and follow their advice (31).
For embedded HE/HP it is postulated that the Hawthorne approach could be feasible and successful for doctors and nurses. The term ‘Hawthorne effect’ has been coined by Henry A. Landsberger when analysing the results from the experiments carried out at the Hawthorne Works, which is a Western Electric factory outside Chicago. The study was aimed to find out how to motivate workers to increase their productivity. The level of light was used as the parameter, which was manipulated and the output measured. The outcome is now a part of the folklore, which showed that no matter how the light had been manipulated (higher, lower, the same) the production went up. At the end of the experiment the production slumped back to precious average.
A number of similar studies followed, using different parameters (break time, payment, colour of walls, changing premises, selecting group members, etc.) with similar results. The conclusion was that just entering a system and singling out the members no matter what manipulation takes place will positively affect the outcome.
It is interesting that one of the major interpretations of the Hawthorne effect was as a contaminating factor in research, which if not accounted for, could distort the findings. The embedded approach, intends to use the Hawthorne effect as the motivational factor for doctors and nurses in dealing with their patients (32).
Some of the elements that affected motivation can be summarized as follows:
– Subject feels that s/he is treated as an individual and not a part of the routine procedure;
– Subject has been taken out of the group and treated as an individual;
– Subject has drawn exclusive attention of the intervention agent;
– Subject is recognized as a special case by the group;
– Subject wants to please the intervention agent;
– Subject receives feed-back on his/her performance;
– Subject treats positive feedback as reward;
All these elements can easily be a part of doctor/nurse – patient interaction, where the patient is treated individually and as a special case, gets recognition through feedback for his/her efforts, and his/her social role is recognized and respected. In conclusion, avoiding lack of personal recognition and a routine approach, should have a positive effect on patients’ motivation and conformity to the doctor/nurse expectations.
THE TRAINING WORKSHOP
The training programme for the health care personnel to meet the new demands of personalized medicine is using the ‘Barić/Osinska Model 2010’ of embedded HE/HP.
The proposed curriculum for training doctors and nurses who are regularly dealing with patients includes the following units and topics:
Unit 1
Topic 1: The concept of personalized medicine; the role of genetic medicine and the new prevention; illness as a psycho-social dysfunction; patient’s roles and forms of health behaviour;
Topic 2: The embedded HP/HE including the methods available; the HP/HE differential diagnosis as the basis for deciding on the contents and the method for individual patients; assessment of the embedded approach:
Unit 2 (33, 34, 35)
Topic 3: Health education methods for the embedded approach: interviewing, counselling, and coaching;
Topic 4: Health promotion methods for the embedded approach: lobbying, advocating, and mediating;
Unit 3
Topic 5: Assessment including prospective impact assessment as a part of the differential diagnosis of the patient;
Topic 6: Evaluation including retrospective impact assessment as a part of the decision about the level of success of the course (36)
Timetable
The workshop for staff dealing with patients in a specific hospital is constructed around several methods of learning. The learning process starts with a formal meeting of participants with the teaching staff. The topics include the concept, the HP/HE methods and the assessment.
After the formal part of the workshop, the participants will be provided with literature related to the embedded approach and be expected to familiarize themselves with this new concept.
The participants will learn how to use each method by following a set of role-playing exercises to be carried out under the supervision of teaching staff. The exercises will use examples from dealing with diabetes and include dealing with anxieties, management techniques, coping abilities and ways of mobilizing social support.
The participants will also carry out a participative evaluation and impact assessment for their personal use and the use by their setting.
Attendance
It has been decided that the testing of this approach will take place in the Department of Internal Medicine and Diabetology, II Medical Faculty, Warsaw Medical University, Brudenowski Hospital. The hospital department has 70 beds and 1000 outpatients. It is run by Professor Dr Jan Taton and 20 doctors and 60 nurses. It is planned to run several courses both for doctors and for nurses, where each course would have between 10-20 participants.
It is planned that separate courses will be offered to doctors and to nurses. This distinction has been made because of the different roles each professional group plays in contact with patients. It is assumed that by sharing the similar competence in methods of approach, their work will be well coordinated and synchronized.
After the initial course it is assumed that if the outcome is successful, additional courses will be offered to the rest of the staff in the Department and that other Departments will want to use a similar approach. In that case it is recommended that the Hospital should develop their in-house trainers, who have been trained for this kind of work. They should be responsible for presenting the proposed curriculum, and for supporting the practical work of the participants and their patients including the assessment.
Method
These workshops should be run on the principles of active participation of the participants, including group and teamwork, the presentation of topics and issues, together with an opportunity for the participants to apply them in simulated exercises. These should be closely linked to the situation and the problems of their patients. The practical work should include role-play and simulations, as well as collecting and analysing data relevant for the participative evaluation of the workshop, and the appraisal of the participants.
The practical exercises should enable the participants to apply embedded HP/HE in their daily working life with the support of members of teaching staff when asked for. The participants should collect information relevant to the assessment of their HE/HP as a value-added contribution to the health gain of the patients.
Outcome
The final outcome should be the use of the acquired competence of the participants in embedded health promotion and health education in their daily work, and assessing the processes and outcomes.
The participants should gain, in addition to competence in using several HE/HP methods, also the confidence that they know what is necessary, and what is possible, in accordance with their specific roles. Special attention should be given to respecting the rights of the patients to avoid the dangers of over-enthusiasm, which could lead to accusations of promoting ‘healthism’ and imposing ‘life-stylism’ on their clients.
Assessment
This should include two aspects: the assessment of the workshop and learning about the methods of assessment of the main study. In the latter case the participants should learn how to assess the value added contribution as a form of embedded HE/HP by the doctors
The proposed workshop for the health care personnel is at present being tested in a Warsaw hospital. It is planned as a three day event including 6 training units. The test includes prospective impact assessment using the Delphi method and a controlled practical implementation. The outcome of this test will be a curriculum which can be applied to all health care settings engaged in introducing personalized medicine (37).
Piśmiennictwo
1. Osińska H, Barić L: Curriculum for embedded HE/HP in a hospital setting, Warsaw 2011. 2. Adler M, Ziglio E (Eds): Gazing into the Oracle: The Delphi Method and its Application To Social Policy and Public Health, Kingsley Publishers, London 2001. 3. Rosenstock I: The Health belief Model and Preventive Behaviour [In:] Becker MH (Ed.): The Health Belief Model and Personal Health Behaviour. Health Education Monographs 1974; 2: 4. 4. Barić L: Recognition of the at-risk role: a means to influence health behaviour [In:] Behaviour Change through Health Education Report of the Int. Seminar on health education, Hamburg 1969, Federal Centre for Health, Cologne 1969. 5. Cartwright D, Zander A: Group Dynamics, Row, Peterson & Co. New York 1953. 6. Connor S: Scientists link defective gene to aggression. The Independent, 26.6.1993. 7. Connor S: Geneticists to fight against blood tests for homosexuality. The Independent, 23.2.1994. 8. Connor S: The Gene Dilemma. The Independent, 6.1.1994. 9. Dawkins R: The Selfish Gene, Oxford University Press, Oxford 1989. 10. Klein J: Working with Groups, Hutchinson & Co. London 1963. 11. Lightfoot L: Flawed gene tests put rape verdicts in doubt. Independent 1994; 8, 5. 12. Newwell J: The new way to put on genes. The Independent, 21.5.1990. 13. Watts S: Ethics of DNA ownership under scrutiny. Independent 1994; 8, 1. 14. Wilkie T: Gene transplants offer hope for sufferes of inherited disorders. The Independent, 16.1.1992. 15. Wilkie T: Scientists investigate the genetic anatomy of human intelligence. The Independent, 11.4.1992. 16. TrofimovY, Kalpana Vora: Gene tests could create European underclass. The European 1993. 17. PricewaterhouseCooper (2009) The new science of personalized medicine, Health Research Institute http://www.pwc.com/personalized medicine (p. 3). 18. Harsanyi Z, Hutton R: Genetic Prophecy – Beyond the Double Helix, Paladin Book, Granada, London 1983. 19. Rennie J: DNA’s new twists. Scientific American, March 1993. 20. Ryan S: Surfing, genes and the meaning of life. The Sunday Times, 15.5.1994. 21. GENOMED.pl, dodatek specjalny z 9 września 2011. Medycyna Spersonalizowana, www.genomed.pl 22. Barić L: Conformity and Deviance in Health and Illness. [In:] Int. Journal of Health Education, Supplement to Vol. XVIII, No. 1, Jan-Mar 1975, Geneva. 23. Barić L, Harrison A: Social Pressure and Health Education, Paper presented at the meeting of Society for Community Medicine and the Rennes School of Public Health, Manchester 1977. 24. Bandura A: Self-efficacy: “Towards a unifying theory of behavioural change?”. Psychological Review 1977; 84(2), 191-215. 25. Barić L: An Introduction to Operational Research in Health Education”, Int. J. Health Education 1968; Vol. XI, No. 2, pp. 50-61. 26. Baric L, Osinska H: Embedded health education and health promotion: A handbook for health professionals, Warsaw 2010. 27. Eysenck MW, Keane MT: Cognitive Psychology, Psychology Press, Hove, UK 2010 (4th edition). 28. WHO (1986) “The Ottawa Charter for Health Promotion” in Health Promotion, No.1, 1986, iii-v. 29. Parsons T: Family Socialization and Interaction Process, Routledge, London (Reprinted 2002). 30. Tones K, Tilford S, Robinson Y: Health Education Effectiveness and Efficiency, Chapman & Hall, London 1990. 31. Atkinson J: A Handbook for Interviewers, HMSO, London 1967. 32. Atkinson JW: Motivational determinants of risk-taking behaviour” in Psychological Review 1957; Vol. 64 (pp. 359-372). 33. Palmer S (Ed.): Handbook of Counselling, Tavistock/Routledge, First published 1989, reprinted 1997, 1998, 1999, 2000. 34. Palmer S (Ed.): Handbook of Counselling, Tavistock/Routledge, First published 1989, reprinted 1997, 1998, 1999, 2000. 35. Whitmore J:Coaching for Performance Nicholas Brealey Publishing, London 2003. 36. Bell DE, Raifa H,Tversky A (eds.): Decision making, Cambridge University Press, Cambridge 1988. 37. http://www.ehfg.org


