Piotr Pasternak, Magdalena Frąckiewicz, *Lidia Zawadzka-Głos
Gentamicin and its ototoxicity
Gentamycyna i jej ototoksyczność
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Wstęp. Stosowanie gentamycyny w okresie noworodkowym jest zaliczane do czynników ryzyka uszkodzenia słuchu. Gentamycyna należy do grupy antybiotyków aminoglikozydowych, które działają głównie na bakterie Gram-ujemne. Ze względu na wysoką skuteczność, niską lekooporność oraz niskie koszty produkcji i leczenia, aminoglikozydy są szeroko stosowane, szczególnie w krajach rozwijających się. Lek ten uszkadza zarówno część ślimakową, jak i przedsionkową. Uszkodzenie słuchu po podaniu gentamycyny jest nieodwracalne.
Cel pracy. Celem pracy była retrospektywna ocena ototoksyczności gentamycyny u dzieci na podstawie kompleksowych badań słuchu.
Materiał i metody. W okresie od stycznia 2019 do czerwca 2020 roku w Klinice Otolaryngologii Dziecięcej UCK WUM hospitalizowano 33 dzieci, które w okresie okołoporodowym były leczone gentamycyną. Wszystkim dzieciom wykonano kompleksową diagnostykę audiologiczną: badanie otoemisji akustycznej, tympanometrię oraz potencjały wywołane z pnia mózgu (BERA).
Wyniki. Spośród 11 dzieci badanych w Klinice u 5 wykazano prawidłową czułość słuchu, u 4 z nich występował przynajmniej jeden dodatkowy czynnik ryzyka uszkodzenia słuchu. W badanej grupie nieprawidłowe wyniki diagnostyki audiologicznej przeprowadzonej w Klinice uzyskano u 7 dzieci.
Wnioski. Należy ograniczyć leczenie gentamycyną do przypadków rzeczywiście życiowo koniecznych. Dzieci leczone gentamycyną w okresie noworodkowym powinny być kierowane na kompleksową diagnostykę słuchu.
Summary
Introduction. The use of gentamicin in the neonatal period is categorized as a risk factor for hearing loss. Gentamicin belongs to a group of aminoglycoside antibiotics that mainly act on Gram-negative bacteria. Due to its high efficacy, low drug resistance, and low production and treatment costs, aminoglycosides are widely used, especially in developing countries. This drug damages both the cochlear and vestibular parts of the inner ear. Hearing damage after gentamicin administration is irreversible.
Aim. The purpose of this study was to retrospectively evaluate gentamicin ototoxicity in children based on comprehensive hearing tests.
Material and methods. Between January 2019 and June 2020, 33 children were hospitalized in the Department of Pediatric Otolaryngology at UCK WUM who were treated with gentamicin in the perinatal period. All children underwent comprehensive audiological diagnostics: acoustic otoemission, tympanometry and auditory brainstem evoked response (BERA).
Results. Among 11 children with incorrect hearing screening test, in 5 the examinations performed in the Clinic showed normal hearing sensitivity, in 4 of them at least one additional risk factor for hearing impairment was present. In the studied group abnormal results of audiological diagnostics performed in the Clinic were obtained in 7 children.
Conclusions. Treatment with gentamicin should be limited to cases that are truly lifethreatening. Children treated with gentamicin in the neonatal period should be referred for a comprehensive hearing diagnosis regardless of the screening results.
Introduction
The Universal Neonatal Hearing Screening Program has been implemented in Poland since 2002. According to the guidelines of this program, 12 risk factors for hearing loss in newborns have been identified. These factors include: known hearing impairment in the family, known congenital malformation of the head and neck, known infection of the TORCH group (toxoplasmosis, rubella, cytomegalovirus, herpes virus, chickenpox, B19 virus), prematurity < 33 weeks of gestation, birth weight < 1500 g, Apgar < 4 w 1 min or < 6 w 5 min, jaundice requiring exchange transfusion, history of cerebrospinal meningitis, stay in intensive care > 7 days, use of mechanical ventilation > 5 days, administration of ototoxic drugs (e.g. gentamicin, vancomycin, meropenem, furosemide, cytostatics etc.), diagnosed congenital malformations associated with hearing loss (1).
One of the risk factors for hearing loss is the use of gentamicin in the neonatal period. Gentamicin belongs to a group of aminoglycoside antibiotics that act mainly on Gram-negative bacteria. Their effect on Gram-positive bacteria is limited, and they have no effect on anaerobic bacteria (2). Due to their high efficacy, low drug resistance, and low production and treatment costs, aminoglycosides (including gentamicin) are widely used, especially in developing countries (3).
Gentamicin is characterized by parenteral absorption and can be given intramuscularly or intravenously. For this reason, it is mainly used in inpatient treatment. Three phases of gentamicin pharmacokinetics are observed. The alpha phase is the distribution of the drug from the blood to the tissues, i.e. until the equilibration of concentrations between them is reached; it lasts up to 30 min. The beta phase is the filtration of the drug in the kidneys (glomerular filtration rate), referred to as the half-life, and lasts from 1.5 to 3 hours. The gamma phase is the excretion of the drug from tissues, including the inner ear, and lasts up to several hundred hours – the length of this phase varies in the literature (2). It is important to note that neonates and preterm infants show increased tissue distribution of gentamicin and have a prolonged half-life of up to 8 hours. Increased tissue distribution also applies to perilymph and endolymph. Prolonged and high blood levels of the drug causes an increased likelihood of hearing damage (4).
Ototoxicity literally means “ear poisoning”. It is damage of the front and/or back of the inner ear after taking a drug, in this case gentamicin. This drug, like other aminoglycosides, damages both the cochlear and vestibular parts of the inner ear. It is believed that the adverse effect of gentamicin on the vestibular part is stronger than on the cochlear part; however, in the neonatal-infant period, it is difficult to objectively assess vestibular pathology (5).
Hearing damage after gentamicin administration is irreversible, it begins with high tones (affecting the basal turn of the cochlea) then progresses to low tones (6). The ototoxicity of gentamicin increases when other hearing-damaging drugs such as glycopeptide antibiotics (vancomycin), loop diuretics (furosemide, etacrynic acid), chemotherapeutics (cisplatin, carboplatin), non-steroidal anti-inflammatory drugs (acetylsalicylic acid, indomethacin). Also the ototoxicity of gentamicin increases in case of glomerular filtration rate disorders resulting from the patient’s other diseases and/or use of nephrotoxic drugs (e.g. polypeptide antibiotics, cyclosporine, tacrolimus). The patient should be cautioned that gentamicin is not recommended. Note that gentamicin is also a nephrotoxic drug (2).
In the last several years, excessive sensitivity of the inner ear as a result of genetic mutations has been put in the foreground. More precisely, it concerns mutations in mitochondrial ribosomal RNA genes, i.e. mutations of the 12 s subunit gene: more often 1555 A > G, less often 1494 C > T. Inheritance of mitochondrial genetic material occurs exclusively through the maternal line, the above problem affects both sexes (7).
The prevalence of the above-mentioned genetic defect is estimated differently, depending on the country, in the USA it occurs in about 1% of the population, in the UK from 2 to 5% of the population (8).
When the mutation is present, there is a greater similarity of mitochondrial rRNA to bacterial rRNA. Gentamicin then binds to this mutant human mitochondrial rRNA disrupting protein synthesis and causing damage to the inner ear – ciliary cells of both types and vascular rod cells (6).
Due to the lack of genetic screening options for the entire neonatal population, alternative ways to prevent hearing loss after gentamicin administration are given great importance:
– taking a detailed history from the parents of a family history of hearing loss,
– monitoring of glomerular filtration rate parameters,
– monitoring antibiotic plasma concentrations,
– thorough audiological diagnostics, including such tests as: otoacoustic emission, BERA, tonal audiometry, high-frequency audiometry.
Ototoxic hearing damage resulting in bilateral sensorineural hearing loss implies impairment of all stages of speech development, leading to both medical and social impairment (8).
Aim
The purpose of this study was to retrospectively evaluate gentamicin ototoxicity in children based on comprehensive hearing tests.
Material and methods
Between January 2019 and June 2020, 33 children were hospitalized in the Department of Pediatric Otolaryngology at UCK WUM who were treated with gentamicin in the perinatal period. The antibiotic was administered at a dose of 4-7 mg/kg body weight once daily. Gentamicin was most frequently administered between the 2nd and 3rd day of life of newborns. The duration of antibiotic administration depended on the severity of infection (fig. 1). In some cases, gentamicin was not the only ototoxic drug used. Four newborns were treated with a combination of gentamicin and vancomycin, two with both vancomycin and meronem and one with vancomycin and furosemide. In the remaining 26 cases, only gentamicin was used from ototoxic drugs. Other risk factors for hearing loss were present in the studied neonates and are presented in table 1.
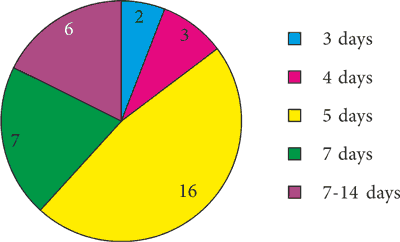
Fig. 1. Duration of antibiotics administration
Tab. 1. Other risk factors for hearing loss
| Risk factors for hearing loss | Numbers of neonates |
| Congenital malformation of the head and neck | 1 (Charge syndrome) |
| Prematurity < 33 weeks of gestation | 14 (24-32 Hbd) |
| Birth weight < 1500 g | 9 (700-1420 g) |
| Apgar < 4 in 1 min or < 6 in 5 min | 5 |
| Cerebrospinal meningitis | 1 |
| Stay in intensive care > 7 days | 11 |
| Use of mechanical ventilation > 5 days | 8 |
All children underwent comprehensive audiological diagnostics: acoustic otoemission study, tympanometry and brainstem evoked potentials response (BERA). The youngest hospitalized child was 3 months old, the oldest 4 years and 3 months. Infants aged 3-6 months were most frequently diagnosed.
Results
All 33 children in the study group had a hearing screening test (acoustic otoemission) performed at birth. In 11 cases the screening test was incorrect; in 8 newborns other risk factors for hearing loss were present. Among 11 children with abnormal hearing screening, 5 had normal hearing, 4 had at least 1 additional risk factor for hearing loss.
In the studied group of 33 children, abnormal results of audiological diagnostics performed in the Clinic were obtained in 7 children. Two of these children had normal hearing screening test results. The exact results of audiological diagnostics are presented in table 2.
Tab. 2. The exact results of audiological diagnostics
| Patient number | Result of hearing screening test | OEA | Result of tympanometry | BERA |
| 1. | Incorrect | Absent | Type As | LE: 500 Hz-50 dB, 1000 Hz-50 dB, click – 40 dB
RE: 500 Hz-60 dB, 1000 Hz-60 dB, click – 50 dB |
| 2. | Incorrect | Absent | Type A | LE: 500 Hz-60 dB, 1000 Hz-60 dB, click – 40 dB
RE: 500 Hz-50 dB, 1000 Hz-50 dB, click – 50 dB |
| 3. | Incorrect | Absent | Type A | LE: 500 Hz-70 dB, 1000 Hz-60 dB, click – 50 dB
RE: 500 Hz-60 dB, 1000 Hz-70 dB, click – 40 dB |
| 4. | Incorrect | Absent | Type A | LE: click – 50 dB
RE: click – 60 dB |
| 5. | Correct | Absent | Type A | LE: click – 50 dB
RE: click – 40 dB |
| 6. | Incorrect | Absent | Type A | LE: 500 Hz-60 dB, 1000 Hz-60 dB, click – 40 dB
RE: 500 Hz-50 dB, 1000 Hz-50 dB, click – 50 dB |
| 7. | Incorrect | Absent | Type A | LE: 500 Hz-60 dB, 1000 Hz-70 dB, click – 70 dB
RE: 500 Hz-70 dB, 1000 Hz-70 dB, click – 60 dB |
Children with abnormal audiological diagnostic results except 1 had other risk factors for hearing loss, table 3.
Tab. 3. Other risk factors for hearing loss in children with abnormal hearing tests
| Patient number | Risk factors for hearing loss
|
| Congenital malformation of the head and neck | Prematurity < 33 weeks of gestation | Birth weight < 1500 g | Apgar < 4 in 1 min or < 6 in 5 min | Stay in intensive care > 7 days | Use of mechanical ventilation > 5 days | Other ototoxic drug |
| 1. | + | | | | | | |
| 2. | | + | | | + | + | |
| 3. | | | | | | | |
| 4. | | + | | | + | + | |
| 5. | | + | + | | + | | |
| 6. | | + | + | + | + | + | |
| 7. | | | | | + | + | + |
Discussion
Gentamicin treatment, although usually effective, implies further problems and questions. The first is clearly the possibility of hearing damage, with all the medical and non-medical consequences of this. It should be noted that the frequency of hearing damage after gentamicin treatment is not well defined. The prevailing opinion is that the problem is rare, but it cannot be ignored.
Another question relates to the duration of follow-up and hearing tests after gentamicin administration, which is not precisely specified.
The question also arises about the total costs of gentamicin treatment, namely whether the costs of further diagnostics and audiological care are not much higher than the costs of treatment alone.
Conclusions
1. Treatment with gentamicin should be limited to cases that are truly life-threatening.
2. Children treated with gentamicin in the neonatal period should be referred for a comprehensive hearing diagnosis regardless of the screening results.
Piśmiennictwo
1. Training and Scientific Conference “Hearing of the newborn”. Poznań 27.09.2019.
2. Hryniewicz W, Meszaros J (red.): Antybiotyki w profilaktyce i leczeniu zakażeń. PZWL, Warszawa 2002: 216, 226, 233.
3. WHO Second Meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines, Geneva 2007.
4. Brunton LL, Lazo JS, Parker KL: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th ed. McGraw Hill Professional 2007: 1243.
5. Selimoglu E: Aminoglycoside – induced ototoxicity. Curr Pharm Des 2007; 13(1): 119-126.
6. Huth ME, Ricci AJ, Cheng AG: Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol 2011; 1-19.
7. Niemczyk K, Jurkiewicz D, Składzień J et al.: Otolaryngologia kliniczna. Wyd. I. Medipage, Warszawa 2015.
8. WHO Second Meeting of the Subcommittee of the Expert Committee on the Selection and Use of Essential Medicines, 2008.
