*Magdalena Sobecka-Frankiewicz1, Ireneusz Nawrot2, Laretta Grabowska-Derlatka3, Agnieszka Mielczarek1
Oral changes in secondary to chronic kidney disease hyperparathyroidism
Zmiany w jamie ustnej wywołane wtórną nadczynnością przytarczyc w przebiegu przewlekłej choroby nerek
1Department of Conservative Dentistry and Endodontics, Medical University of Warsaw, Poland
Head of Department: Professor Agnieszka Mielczarek, MD, PhD
2Department of General, Vascular and Transplantation Surgery, Medical University of Warsaw, Poland
Head of Department: prof. Sławomir Nazarewski MD, PhD
32nd Department of Radiology, Medical University of Warsaw, Poland
Head of Department: prof. Olgierd Rowiński MD, PhD
Streszczenie
Najczęstszą przyczyną wtórnej nadczynności przytarczyc jest przewlekła choroba nerek. Zaburzenie równowagi pomiędzy wapniem, fosforem i witaminą D3 w przebiegu wtórnej nadczynności przytarczyc może wpływać na stan zdrowia jamy ustnej. Zmiany te objawiają się w kościach szczęk jako utrata gęstości kości, ogniska demineralizacji, a nawet jako duże guzy zniekształcające okolicę twarzoczaszki – guzy brunatne, włókniaki kostniejące i torbiele kostne. Obserwuje się również zwiększone występowanie próchnicy, chorób przyzębia i infekcji grzybiczych. Wtórna nadczynność przytarczyc w młodym wieku może powodować zaburzenia rozwoju zębów i nieprawidłowości zębowe. Leczenie stomatologiczne u pacjenta z wtórną nadczynnością przytarczyc może być wyzwaniem dla lekarza stomatologa, dlatego konieczna jest ścisła współpraca z lekarzem prowadzącym, a także jak najszybsze wprowadzenie profilaktyki stomatologicznej. Dodatkowo, ze względu na występowanie charakterystycznych zmian w jamie ustnej, stomatolog może odegrać istotną rolę w pierwotnym wykrywaniu wtórnej nadczynności przytarczyc.
Summary
The most common cause of secondary hyperparathyroidism is a chronic kidney disease. The imbalance between calcium, phosphorus and vitamin D3 in the course of secondary hyperparathyroidism may affect an oral health. The changes are presented in jaw bones as a loss of bone density, focuses of demineralization or even large tumors deforming facial region – brown tumors, ossifying fibromas and aneurismal bone cysts. There is also a higher frequency of dental caries, periodontal disease and fungal infections. Secondary hyperparathyroidism in the young age may cause teeth development disorders and dental abnormalities. Dental treatment in patient with secondary hyperparathyroidism can be a challenge for a dentist, so the strict cooperation with an attending physician is needed, as well as to introduce dental prophylactic procedures as soon as possible. In addition, due to the occurrence of characteristic changes in the oral cavity, the dentist may play an important role in the primary detection of secondary hyperparathyroidism.
Introduction
Hyperparathyroidism occurs in about 0.05 to 0.10% of the population. Mostly affects women (3 times more often than men), mainly in middle age (30-60 years) (1-3). Hyperparathyroidism is a condition connected with an overproduction of parathyroid hormone that increases calcium concentration in the blood by potentiating its absorption from bones and kidneys. Secondary hyperparathyroidism is an over-secretion of parathyroid hormone caused by a decreased level of blood calcium. The most common cause is a chronic renal insufficiency, which is estimated at 8-16% of the population (4, 5). Other causes may relate to other kidney diseases, malabsorption syndrome, and reduced vitamin D3 production (2, 3). The basis for secondary hyperparathyroidism is the insufficient conversion of vitamin D3 to its active form the lack of second hydroxylation of cholecalciferol in renal tubules by 1-alpha-hydroxylase (OH) D3 and inadequate removal of phosphates by inefficient kidneys, what as a consequence decreases the serum calcium level. Compensatory the parathyroid size and hormone secretion is increased and hyperparathyroidism occurs (6). Parathyroid hormone works primarily on the bones and kidneys, where it causes increased calcium absorption and additionally in kidneys reduces the phosphorus absorption and increases production of the active form of vitamin D3 (1, 7, 8). In the case of secondary hyperparathyroidism caused by chronic kidney disease, the onset of the disorder is associated with changes in calcium, vitamin D and phosphate levels what affects the bones and soft tissues. Therefore, are often defined as the CKD-MBD syndrome (chronic kidney disease – mineral and bone disorder syndrome) (9). Another syndrome, described in secondary hyperparathyroidism, is the Sagliker syndrome, noticed for the first time in severe chronic kidney disease. Its frequency among people with chronic kidney disease is about 0.5%. Its occurrence is associated with the destructive effect of hyperparathyroidism at a young age. This syndrome includes: facial deformity, short stature, jaw bone lesions, teeth malocclusion – 2nd class, dental abnormalities, fingertip changes, scapula and collarbone deformities and hearing, neurological and psychological disorders. There is also a higher rate of early mortality (10-15).
Due to the influence of calcium, phosphorus and vitamin D3 on oral health, their imbalance in the course of secondary hyperparathyroidism can be important in functioning masticatory system. In addition, the impact of hyperparathyroidism on the dysfunction of internal organs may also be presented as changes in the oral cavity.
Symptomatology
The earliest symptoms in the oral cavity are jaw bone lesions. In most cases the changes are painless and accidentally found on extra- and intraoral radiographs, although sometimes patients report vague discomfort in the mandibular area, rarely in the maxilla (2, 16, 17) (fig. 1-4).
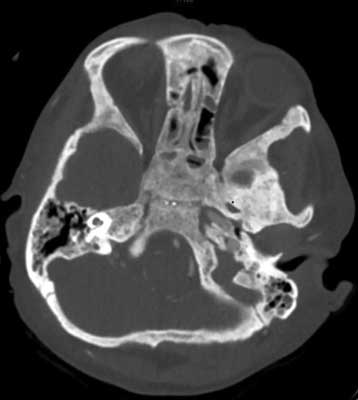
Fig. 1. CT exam, transverse projection. Bone changes – osteosclerosis present in cranial basis area
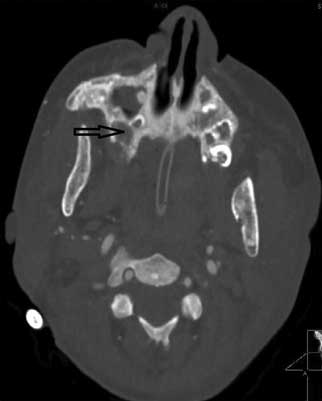
Fig. 2. CT exam, transverse projection of maxilla. Osteosclerosis and erosions (arrow)
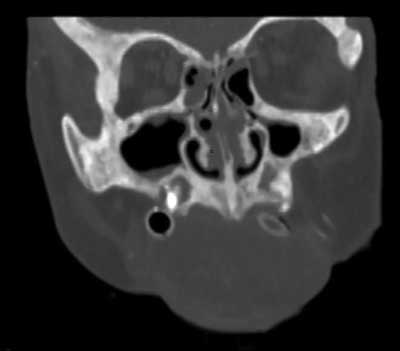
Fig. 3. CT exam, coronal projection of maxilla. Bone changes – osteosclerosis
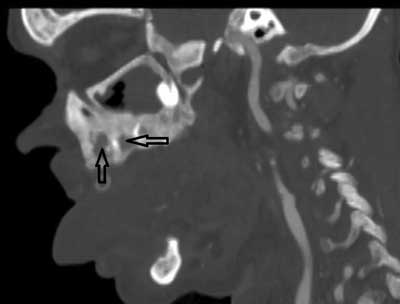
Fig. 4. CT exam, sagital projection of maxilla. Bone changes: osteosclerosis and erosions (arrows)
There is a generalized loss of bone density that results in blurring of the characteristic trabecular pattern with an appearance of ground glass (18-21). In about 10% of patients with hyperparathyroidism there is also a loss of lamina dura of the jaw bone. Rarely, jaw hyperplasia and jaw and facial bones enlargement may occur (1, 9, 16, 22).
Changes in the bones have a direct effect on the teeth, causing gradual loosening, displacement, and eventually tooth loss (1, 2, 23, 24). Malocclusion and chewing difficulties appear due to teeth’s hypersensitivity to occlusal forces and percussion. The influence of secondary hyperparathyroidism on the dentition is also seen in the x-rays as a narrowing and calcifications in the pulp chambers as well as denticuli inside it. There is also seen enlargement of the periodontal ligament around the roots what visibly correlates with parathyroid hormone levels (2-4, 22, 25, 26). Hyperparathyroidism is also a favorable factor of occurrence, especially multiple, of internal and external resorptions of the teeth (27, 28).
Hyperparathyroidism may be manifested by changes in the development of the dentition, such as alterations in dental eruption or dental abnormalities. Studies show that modifications of calcium, phosphorus, and parathyroid hormone levels can contribute to the defects in the formation of the teeth and alveolar processes (2, 29).
High levels of parathyroid hormone can affect periodontal tissues and may worsen the course of periodontal disease by increasing the production of pro-inflammatory cytokines (30-32). However, some studies indicate for the lack of significant effects of secondary hyperparathyroidism on periodontal disease indicators and radiologically evaluated alveolar bone level (30, 33).
Secondary hyperparathyroidism is associated with a presence of single or multiple focuses of bone osteolysis. They are most commonly found as brown tumors, in advanced form called osteitis fibrosa cystica or von Racklinghausen’s bone disease (21, 26, 34). These lesions usually appear as a late manifestation of the disease. Brown tumor is a focal, sinus-like bone destruction with the presence of caverns filled with connective tissue. Coalescence of bone lesions with bone marrow cavities causes bleeding and thus their brown coloration by blood hemosiderin (19, 21, 35, 36). Histologically, the brown tumor may be indistinguishable from the peripheral gigantocellularis granuloma (19, 37). It may achieve extreme sizes that may be mimicking the brown tumor is found in 1.5-1.7% of patients with secondary hyperparathyroidism (1, 4, 7), but also, even more likely (4.5%), may affect patients with primary hyperparathyroidism (7, 38, 39). In most cases, equalizing of parathyroid hormone levels cause spontaneous withdrawal of the tumor (36, 37, 40), but in some cases, despite the hormonal regulation, there is still need for surgical resection of the lesion (2, 22). Corticosteroids are also used to reduce the tumor mass before its resection (7).
Osteitis fibrosa cystica can cause spontaneous pain and palpation sensitivity of the bone, as well as its shape changes and even large, deteriorating aesthetics deformations of the facial area. It can also cause the pathological fractures of the mandible, the displacement or resorption of the teeth’s roots and by its growth exert the pressure on neighboring structures (2, 3, 9, 16, 24). The brown tumor in the facial area is most often located in the mandible, rarely in the maxilla, most often occurs in the part of premolars and molars (2, 26).
Among other described bone lesions that may occur in secondary hyperparathyroidism in the facial region and should be differentiated with brown tumor are:
– aneurysmal bone cyst,
– ossifying fibroma.
Ossifying fibroma in secondary hyperparathyroidism is very rare (36), more commonly found in primary hyperparathyroidism (5). Most commonly occurs in 3-4 decade of life, regardless of gender, as a painless, irregular lesion with both radiopacities and radiolucencies and with the characteristic sclerotic rim seen on the radiographs (1, 35, 36, 41).
Similarly to brown tumor, it most often appears in the mandibular jaw in the area of premolars and molars. Sometimes ossifying fibroma can have a large size entering the maxillary sinus or pressing on the orbital floor, causing pain, parenthesis, and even vision disorders. Rarely causes resorption of the roots of the teeth. In the mouth it becomes visible as a whitish hard mass that can dislocate the teeth and cause a disproportion of the face. Ossifying fibroma has a slight chance of malignant transformation, estimated to be below 0.5%. In practice, it is difficult to distinguish ossifying fibroma from brown tumor, and definitive diagnosis is based on histopathological examination (1, 32, 35, 41, 42).
Aneurysmal bone cyst is rarely found – in about 4% of the population and in 1.9-2% may be located in the jaw bones. Most often occurs in 2 decades of life, regardless of gender. It can occur as a primary change or as a transformation of ossifying fibroma, displasia fibrosa or granuloma gigantocellularis. It is an enlargement of the bone with its periosteum, containing inside numerous connective tissue divisions, blood vessels, blood clots and bone trabeculae. Rupture of aneurysmal bone cyst causes massive hemorrhage. Due to the enlargement and compression on surrounding structures, it can cause facial disproportion, pain and neurological symptoms (43).
Among other described changes in the area of the oral cavity in hyperparathyroidism are also calcium deposits in soft tissues, especially in salivary glands (2, 3, 9, 18, 36).
Some studies also report an increased incidence of torus palatinus or torus mandibularis in secondary hyperparathyroidism, although they are not explicitly confirmed (44, 45).
Hyperparathyroidism is considered as a predisposing factor for oral candidiasis. It may be found as a chronic hyperplastic candidiasis (fungal leukoplakia) that is characterized by the presence of white plaques or pellets infiltrated superficially by C. albicans (46).
The above-described oral lesions may be an important indicator of secondary hyperparathyroidism, but due to their non-specificity they should be carefully differentiated from other diseases (e.g. primary hyperparathyroidism, celiac disease) (38, 47).
Dental care in patients with secondary to chronic kidney disease hyperparathyroidism
The condition of the oral health in secondary hyperparathyroidism may be related to its main cause – chronic kidney disease. Many oral pathologies may be caused or aggravated by poor general condition of the patient and co-existing pathologies such as immune deficiency and oxidative stress. In patients with chronic renal failure, there is an increase in the incidence of periodontal disease, which may be associated with immune deficiencies, vitamin D3 deficiency, uremic toxemia, anemia, malnutrition, osteoporosis and other bone metabolism disorders, insulin resistance or diabetes mellitus, hepatic disorders in the course of frequent viral infections and poor general condition decreasing the ability of maintaining good oral hygiene (7, 32, 48).
In patients with chronic kidney disease, the condition of the dentition is worse than in the general population. Increased exposure to tooth decay and tooth loss is associated with a decreased salivary secretion and its increased density. In addition, the occurrence of high levels of urea in saliva causes an increase in saliva pH, what accelerates the mineralization of the bacterial plaque, formation of calculus deposits and compromises oral hygiene. Reduction of saliva secretion causes mucosa dryness and thus increased exposure to fungal infections and the presence of prosthetic stomatopathy (7, 29, 32, 48).
Dental care of patients with secondary hyperparathyroidism can be a challenge for the dentist. Changes in the oral cavity can be a consequence of the imbalance between calcium, phosphorus and vitamin D3 and the effect of chronic renal failure as the leading cause of secondary hyperparathyroidism.
Patients with secondary hyperparathyroidism have higher problems with maintaining proper oral hygiene, mainly due to salivary gland dysfunction (salivary gland calcifications compromising their function, decreased salivary flow, increased density of saliva), a poor general health condition impairing manual skills and other dysfunctions coexisting with chronic kidney disease (48). A significant role may play also a decrease in vitamin D3 levels, which, except from to the effects on calcium metabolism, also has antimicrobial properties against bacteria involved in cariogenic process and periodontal inflammation (by activation of immune cells: B- and T-lymphocytes, macrophages and monocytes and stimulation of the production of antibacterial peptides: cathelicidin and beta-defensin) (49-51).
These conditions contribute to an increased risk of dental caries and periodontal disease (3, 26, 29, 52). Concerning this, it is particularly important to emphasize dental prophylaxis by introducing a proper tooth brushing techniques, using supplementary instruments such as dental floss, interdental brushes (especially for teeth gaps) as well as mouthwashes. Fluoride prophylaxis or based on other formulas (for example based on casein’s compounds) may also be important. There is also a need for systematic control visits, combined with the removal of plaque and calculus deposits (46, 52). A proper periodontal and dental treatment, by reducing the general inflammation, tend to improve the status of chronic renal disease (53).
The progress of caries can lead to pulpitis requiring endodontic treatment. Narrow tooth chambers, obliterated root canals, calcifications of the pulp, and frequent occurrence of internal and external root resorption can significantly impede the root canal treatment and promote the occurrence of its complications (27, 28, 54). In the context of endodontic treatment in patients with hyperparathyroidism, attention should also be paid to the difficulty in making the correct diagnosis. The focuses of bone demineralization in osteitis fibrosa seen on the radiographs, may overlap the apical parts of the root of the teeth and can mimic the changes connected with apical periodontitis. Decisions about endodontic treatment of such teeth are taken inadequately (2, 3, 55, 56).
In the secondary hyperparathyroidism, due to demineralization of the jaw bones, there is a mobility and displacement of the teeth, what leads to malocclusion (2-4, 26). It can cause difficulties in chewing, speech, and hygiene procedures as well as tempomandibular joint disorders. Stiffness of the tempomandibular joint may additionally aggravate these discomforts (2).
Drifting and gradual teeth loss are indications for prosthetic rehabilitation of the patients. Because of salivary gland dysfunction in patients with secondary hyperparathyroidism, the adhesion of the removable prosthesis is weak, resulting in a greater tendency for oral mucosal microinjuries and prosthetic stomatopathy (6, 26, 46). There is also a higher tendency for fungal infections of the mucosa in the oral cavity. Difficulties with fitting prosthetic restorations may also be associated with continuous changes in the form of the bone due to its loss, as well as with tooth decay and tooth loss (they do not provide proper support for prosthesis). Focuses of demineralization in the bone may also disable dental implants placing, but proper general treatment leading to remission of changes again enables this possibility (57).
Extractions and all procedures involving the injury of soft tissues, in patients with hyperparathyroidism should be performed in antibiotic cover. Currently, the standard recommended dental regimen is 2 g amoxicillin or amoxicillin with clavulonic acid, alternatively 0.6 g clindamycin 1 hour prior to surgery, however, due to the renal toxicity of these above mentioned antibiotics, in patients with secondary hyperparathyroidism should be used carefully and with an agreement of attending physician (1, 29, 58, 59).
Another problem to be considered is the use of anticoagulants by patients undergoing dialysis. Anticoagulants may cause increased bleeding during surgery, so consulting an attending physician before starting treatment is needed (1). In addition, treatment of dialysis patients should be best performed the day after dialysis, due to patient’s best general condition on that day (29).
Especially difficult to treat can be a patient with Sagliker syndrome. Due to numerous changes in the masticatory system, a dentist may be an important member of a multidisciplinary medical team in the care of such a patient.
Conclusions
Patients with secondary to chronic kidney disease hyperparathyroidism, in addition to specialist treatment, should also be under the care of a dentist. Frequent controls, prophylaxis and treatment of oral diseases help to maintain good oral health, what leads to a proper general condition of the patient. Due to the occurrence of characteristic changes in the oral cavity, the dentist may also play an important role in the detection of secondary hyperparathyroidism (2). Among specialists in contact with hyperparathyroid patients, dental surgeons are the ones who most frequently refer to endocrinologists (38). Particularly important are radiological examinations performed in dentistry such as orthopantomogram, or even single intraoral apical radiograph, on which the focuses of bone demineralization can be identified. The plural or large lesions that appear in the jaw bones may direct to search for endocrine causes of their occurrence (16, 19, 56, 60). Use of CBCT imaging allows more accurate analysis of the lesion, with the possibility of preliminary diagnosis (4, 61).
Piśmiennictwo
1. Kakade SP, Gogri AA, Umarji HR, Kadam SG: Oral manifestations of secondary hyperparathyroidism: A case report. Contemporary Clinical Dentistry 2015; 6(4): 552-558.
2. Mittal S, Gupta D, Sekhri S, Goyal S: Oral manifestations of parathyroid disorders and its dental management. J Dent Allied Sci 2014; 3: 34-38.
3. Khalekar Y, Zope A, Brahmankar U, Chaudhari L: Hyperparathyroidism in dentistry: Issues and challenges!! Indian J Endocrinol Metab 2016; 20(4): 581-582.
4. Çağlayan F, Dağistan S, Keleş M: The osseous and dental changes of patients with chronic renal failure by CBCT. Dentomaxillofac Radiol 2015; 44(5).
5. Jha V, Garcia-Garcia G, Iseki K et al.: Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382(9888): 260-272.
6. Karwacki JH, Skalski A, Nawrot I et al.: Analiza wyników badań histopatologicznych usuniętych gruczołów przytarczowych u pacjentów operowanych z powodu wtórnej nadczynności przytarczyc. Adv Clin Exp Med 2005; 14(2): 217-223.
7. Olczak-Kowalczyk D, Kosiorowska-Bednarczyk A, Stpa Z et al.: Guz brunatny żuchwy i szczęki w przebiegu wtórnej nadczynności przytarczyc u pacjenta po przeszczepieniu nerki – własne obserwacje. Czas Stomatol 2009; 62(10): 816-823.
8. Zawierucha J, Małyszko J, Małyszko J et al.: Współczesne poglądy na diagnostykę i leczenie wtórnej nadczynności przytarczyc. Przeg Lek 2016; 7: 497-503.
9. Raubenheimer EJ, Noffke CE, Mohamed A: Expansive jaw lesions in chronic kidney disease: review of the literature and a report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 119(3): 340-345.
10. Yavascan O, Kose E, Alparslan C et al.: Severe renal osteodystrophy in a pediatric patient with end-stage renal disease: Sagliker syndrome? J Ren Nutr 2013; 23(4): 326-330.
11. Sagliker Y, Acharya V, Ling Z et al.: International study on Sagliker syndrome and uglifying human face appearance in severe and late secondary hyperparathyroidism in chronic kidney disease patients. J Ren Nutr 2008; 18(1): 114-117.
12. Sagliker Y, Balal M, Sagliker Ozkaynak P et al.: Sagliker syndrome: uglifying human face appearance in late and severe secondary hyperparathyroidism in chronic renal failure. Semin Nephrol 2004; 24(5): 449-455.
13. Wu W, Qian L, Chen X, Ding B: A case of Sagliker syndrome and literature review. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2014; 39(10): 1100-1104.
14. Mohebi-Nejad A, Gatmiri SM, Abooturabi SM et al.: Diagnosis and treatment of Sagliker syndrome: a case series from Iran. Iran J Kidney Dis 2014; 8(1): 76-80.
15. Panezai MA, Ahmed S, Colbert GB: Sagliker syndrome in a patient with end-stage renal disease with secondary hyperparathyroidism. Proc (Bayl Univ Med Cent) 2019; 32(4): 624-626.
16. Antonelli JR, Hottel TL: Oral manifestations of renal osteodystrophy: case report and review of the literature. Spec Care Dentist 2003; 23(1): 28-34.
17. Chi AC, Damm DD, Neville BW et al.: Oral and Maxillofacial Pathology. 3rd ed. Saunders 2009: 839.
18. Azzi L, Cimetti L, Annoni M et al.: A Giant-Cell Lesion with Cellular Cannibalism in the Mandible: Case Report and Review of Brown Tumors in Hyperparathyroidism. Case Rep Dent 2017; 2017: 9604570.
19. Preeti D, Chaya DM, Keerthi G: Radiographic manifestations of systemic diseases in jaw bones: A systemic review. Asian Pac J Health Sci 2014; 1(2): 120-130.
20. Roleder J, Wilczyńska-Borawska M, Nowosielski C, Małyszko J: Interdyscyplinarny charakter chorób jamy ustnej – implikacje kliniczne. Przegląd Lekarski 2016; 73(4): 233-237.
21. Singhal AA, Baijal SS, Sarin D, Pathak A: Unusually Large Brown tumor of Mandible in a Case of Secondary Hyperparathyroidism Mimicking Cherubism. Indian J Nucl Med 2018; 33(2): 132-135.
22. Regezi JA, Sciubba JJ, Jordan RCK: Oral Pathology: Clinical Pathologic Correlations. 7th ed. Elsevier 2017: 349.
23. Queiroz IV, Queiroz SP, Medeiros R et al.: Brown tumor of secondary hyperparathyroidism: surgical approach and clinical outcome. Oral Maxillofac Surg 2016; 20: 435.
24. Davis EM: Oral Manifestations of Chronic Kidney Disease and Renal Secondary Hyperparathyroidism: A Comparative Review. J Vet Dent 2015; 32(2): 87-98.
25. Yang CY, Chang ZF, Chau YP et al.: Uremia Induces Dental Pulp Ossification but Reciprocally Inhibits Adjacent Alveolar Bone Osteogenesis. Calcif Tissue Int 2015; 97(5): 466-475.
26. Fabue I LC, Soriano YJ, Perez MGS: Dental management of patients with endocrine disorders. J Clin Exp Dent 2010; 2(4): 196-203.
27. Nagaraj E, Kaur RP, Raghuram PH, Kumar PS: Multiple internal resorption in permanent teeth associated with hyperparathyroidism. Indian J Dent Res 2013; 24(1): 128-131.
28. Arabska-Przedpełska A, Pawlicka H: Współczesna endodoncja w praktyce. Wydanie II poprawione. Bestom, Łódź 2012: 396-397.
29. Scully C: Scully’s medical problems in dentistry. 7th ed. Churchill Livingstone, Elsevier 2014: 342-343.
30. Lütfioğlu M, Sakallioğlu U, Sakallioğlu EE et al.: The impact of dietary induced hyperparathyroidism on healthy and diseased periodontia: an experimental study in rats. J Clin Periodontol 2012; 39(3): 264-271.
31. Lütfioğlu M, Sakallioğlu U, Sakallioğlu EE et al.: Dietary-induced hyperparathyroidism affects serum and gingival proinflammatory cytokine levels in rats. J Periodontol 2010; 81(1): 150-157.
32. Wyganowska-Świątkowska M: Rola pozostałości komórek nabłonkowej pochewki korzenia w procesie regeneracji przyzębia – przegląd literatury. Przeg Lek 2015; 10: 581-583.
33. Frankenthal S, Nakhoul F, Machtei EE et al.: The effect of secondary hyperparathyroidism and hemodialysis therapy on alveolar bone and periodontium. J Clin Periodontol 2002; 29(6): 479-483.
34. Sun W, Sun W, Liu J et al.: Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development 2010; 137(6): 985-992.
35. Kruś S, Skrzypek-Fakhoury E: Patomorfologia kliniczna. Wyd. III. PZWL, Warszawa 2007: 387, 795, 807, 808.
36. Devresse A, Raptis A, Claes A-S, Labriola L: A Swelling in the Mouth in a Chronic Hemodialysis Patient. Case Reports in Nephrology 2016; 2016: 4970702.
37. Zissimos M, Goldberg H, Sinson G et al.: Final Diagnosis – Brown Tumor (Giant Cell Tumor of Hyperparathyroidism). University of Pittsburgh School of Medicine. Retrieved 2008: 11-17.
38. Jodkowska A, Tupikowski K, Szymczak J et al.: Interdisciplinary Aspects of Primary Hyperparathyroidism: Symptomatology in a Series of 100 Cases. Adv Clin Exp Med 2016; 25(2): 285-293.
39. Karwacki JH, Skalski A, Nawrot I: Analysis of Prior Medical Histories of Patients Operated on for Primary Hyperparathyroidism. Adv Clin Exp Med 2007; 16(2): 257-262.
40. Mellouli N, Belkacem Chebil R, Darej M et al.: Mandibular Osteitis Fibrosa Cystica as First Sign of Vitamin D Deficiency. Case Rep Dent 2018; 2018: 6814803.
41. Du Preez H, Adams A, Richards P, Whitley S: Hyperparathyroidism jaw tumour syndrome: a pictoral review. Insights into Imaging 2016; 7(6): 793-800.
42. Lipczyński K, Pokrowiecki R, Zaleska M, Urbańczyk K: Peripheral ossifying fibroma – case report. J Stoma 2015; 68(2): 226-232.
43. Bakhtiari S, Bakhshi M, Mashhadiabbas F et al.: Bimaxillary Aneurismal Bone Cyst in Patient with End Stage Renal Disease and Hyperparathyroidism: A Rare Case Report and Review of the Literature. Case Reports in Dentistry 2016.
44. Sisman Y, Gokce C, Sipahioglu M et al.: Torus palatinus in end-stage renal disease patients receiving peritoneal dialysis: Does renal osteodystrophy play a role? J Dent Sci 2012; 7(2): 154-158.
45. Hsu CL, Hsu CW, Chang PC et al.: Oral Tori in Chronic Peritoneal Dialysis Patients. PLoS One 2016; 11(6).
46. Petkowicz B, Skiba-Tatarska M, Wysokińska-Miszczuk J: Kandydoza jamy ustnej. Gerontol Pol 2006; 14(4): 160-164.
47. Krzywicka B, Herman K, Kowalczyk-Zając M, Pytrus T: Celiac disease and its impact on the oral health status – review of the literature. Adv Clin Exp Med 2014; 23(5): 675-681.
48. Wilczyńska-Borawska M, Małyszko J, Stokowska W: Stan uzębienia i przyzębia u pacjentów z przewlekłą chorobą nerek. Postepy Nauk Med 2010; 3: 233-237.
49. Grant WB: A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol 2011; 3(3): 193-198.
50. He CS, Fraser WD, Tang J et al.: The effect of 14 weeks of vitamin D3 supplementation and antimicrobial peptides and proteins in athletes. J Sports Sci 2015; 34(1): 67-74.
51. Rathee M, Singla S, Kundu R: Vitamin D and Oral Health: A review. Medical Science Oct 2013; 2: 10.
52. Jańczuk Z (red.): Stomatologia zachowawcza. Zarys kliniczny. Podręcznik dla studentów stomatologii. Wydanie IV. PZWL, Warszawa 2014: 160-184, 254.
53. Delbove T, Gueyffier F, Juillard L et al.: Effect of periodontal treatment on the glomerular filtration rate, reduction of inflammatory markers and mortality in patients with chronic kidney disease: A systematic review. PLoS One 2021; 16(1): e0245619.
54. Kwak EJ, Oh KY, Perinpanayagam H, Kum KY: Internal Resorption of Multiple Posterior Teeth in a Patient Diagnosed with Hyperparathyroidism: A Case Report. J Endod 2021: S0099-2399(21)00257-0.
55. Loushine RJ, Weller RN, Kimbrough WF, Liewehr FR: Secondary hyperparathyroidism: a case report. J Endod 2003; 29(4): 272-274.
56. Jalali P, Kim SG: Multiple periradicular radiolucencies mimicking endodontic lesions in renal osteodystrophy of the mandible: a case report. Int Endod J 2016; 49(7): 706-714.
57. Brabyn P, Capote A, Belloti M, Zylberberg I: Hyperparathyroidism Diagnosed Due to Brown Tumors of the Jaw: A Case Report and Literature Review. J Oral Maxillofac Surg 2017.
58. Napora M: Profilaktyka antybiotykowa pacjentów z grupy wysokiego ryzyka wystąpienia infekcyjnego zapalenia wsierdzia w stomatologii. Nowa Stomatol 2008; 1: 24-26.
59. Buczkowska-Radlińska J, Lipski M: Profilaktyka i leczenie stomatologiczne osób z zaburzeniami i chorobami ogólnymi. Wydawnictwo Pomorskiego Uniwersytetu Medycznego w Szczecinie 2011: 31.
60. Munhoz EA, Cardoso CL, Capelozza AL et al.: Panoramic radiography and its role in the diagnosis of systemic disorders. Gen Dent 2010; 58(1): 46-49.
61. Kanjevac T, Bijelic B, Brajkovic D et al.: Impact of Chronic Kidney Disease Mineral and Bone Disorder on Jaw and Alveolar Bone Metabolism: A Narrative Review. Oral Health Prev Dent 2018; 16(1): 79-85.



