Forty-two consecutive patients (19 men and 23 women) with SAH, admitted to the neurosurgery clinic of our hospitals between February 1998 and December 2002, were included in the study (Table 1). Their age ranged from 17 to 80 years (mean 57.2 ±11.9, median 58). Inclusion criteria were: a) SAH proven by CT examination which occurred within 24 hours from admission, b) age between 17 and 80 years, c) a good clinical condition (Glasgow Coma Score = 12, Hunt and Hess grade
Table 1. Patient characteristics, risk factors, DSA findings, SPECT findings, kind of treatment, clinical course and a 6-month outcome.
| Patient No | Sex | Age | H&H grade | GCS on admission | CT (Fisher grade) | DSA (Site of the aneurysm) | SPECT score (SPDS) | SPECT grade | Treatment | Clinical Vasospasm (clinical findings) | Outcome (GOS) |
| 1 | M | 17 | I | 14 | 2 | neg | 12 | 2 | conservative | Yes (confusion, visual impairment) | 5 |
| 2 | M | 40 | III | 13 | 3 | pos (ACA) | 17 | 3 | surg clip ? VPS | Yes (right VII cranial nerve palsy) | 4 |
| 3 | M | 75 | III | 14 | 2 | neg | 7 | 1 | conservative | No | 4 |
| 4 | F | 59 | II | 14 | 2 | pos (ACA) | 1 | 1 | surg clip | No | 5 |
| 5 | F | 74 | I | 15 | 3 | pos (PCA L+MCA R) | 14 | 2 | surg clip | Yes (confusion, left hemiparesis) | 1 |
| 6 | F | 80 | II | 14 | 3 | not performed | 10 | 2 | conservative | Uncertain | 1 |
| 7 | M | 80 | III | 14 | 4 | pos (ACA) | 15 | 2 | endov coil | Yes (confusion, left hemiparesis) | 1 |
| 8 | F | 32 | II | 15 | 2 | neg | 13 | 2 | conservative | No | 5 |
| 9 | M | 59 | II | 14 | 3 | neg | 22 | 3 | VPS | Uncertain | 1 |
| 10 | F | 69 | II | 14 | 2 | neg | 17 | 3 | conservative | Yes (confusion, dysarthria, visual impairment) | 5 |
| 11 | M | 72 | II | 14 | 3 | pos (MCA L) | 10 | 2 | surg clip | No | 1 |
| 12 | F | 52 | II | 15 | 3 | neg | 17 | 3 | VPS | Uncertain | 3 |
| 13 | M | 45 | II | 13 | 3 | pos (MCA L) | 12 | 2 | surg clip | Uncertain | 5 |
| 14 | F | 62 | II | 15 | 2 | neg | 3 | 1 | conservative | No | 5 |
| 15 | M | 55 | II | 14 | 2 | pos (BA) | 11 | 2 | endov coil ? surg clip | Yes (confusion, left leg hypokinesis) | 3 |
| 16 | F | 47 | II | 15 | 2 | neg | 1 | 1 | conservative | No | 5 |
| 17 | F | 60 | I | 15 | 3 | neg | 21 | 3 | VPS | Uncertain | 4 |
| 18 | F | 71 | II | 15 | 2 | pos (ACA) | 12 | 2 | surg clip ? VPS | Yes (left cranial nerve III palsy) | 3 |
| 19 | M | 60 | I | 15 | 2 | pos (ACA) | 5 | 1 | surg clip | No | 5 |
| 20 | F | 60 | II | 15 | 2 | neg | 7 | 1 | conservative | No | 5 |
| 21 | F | 57 | II | 14 | 2 | pos (IC) | 14 | 2 | endov coil | No | 5 |
| 22 | M | 47 | III | 12 | 3 | pos (ACA) | 16 | 2 | surg clip | Yes (drowsiness, confusion) | 1 |
| 23 | F | 56 | II | 14 | 2 | neg | 13 | 2 | conservative | Yes (headache, drowsiness, left leg hypokinesis) | 5 |
| 24 | M | 41 | II | 15 | 2 | pos (ACA) | 10 | 2 | surg clip | No | 4 |
| 25 | F | 53 | II | 14 | 3 | pos (MCA R) | 14 | 2 | surg clip | Yes (confusion, left hypokinesis) | 3 |
| 26 | M | 47 | II | 14 | 2 | neg | 7 | 1 | conservative | No | 5 |
| 27 | F | 46 | II | 14 | 2 | neg | 4 | 1 | conservative | No | 5 |
| 28 | M | 69 | II | 14 | 4 | neg | 5 | 1 | conservative | No | 5 |
| 29 | M | 53 | II | 13 | 2 | pos (MCA R) | 15 | 2 | endov coil | Yes (Confusion) | 4 |
| 30 | F | 52 | I | 15 | 2 | neg | 7 | 1 | conservative | No | 1 |
| 31 | M | 68 | II | 15 | 4 | neg | 13 | 2 | conservative | No | 1 |
| 32 | F | 68 | II | 14 | 3 | pos (MCA R) | 13 | 2 | surg clip | Yes (confusion, left hemiparesis) | 4 |
| 33 | M | 69 | II | 14 | 4 | pos (ACA) | 19 | 3 | conservative | Yes (aphasia, right hemiparesis) | 1 |
| 34 | F | 64 | II | 14 | 3 | pos (PCA L) | 16 | 2 | surg clip ? VPS | Yes (confusion, anisocoria) | 3 |
| 35 | F | 38 | II | 14 | 2 | neg | 2 | 1 | conservative | No | 5 |
| 36 | M | 70 | II | 15 | 2 | neg | 7 | 1 | VPS | Uncertain | 5 |
| 37 | F | 49 | III | 14 | 4 | pos (MCA R) | 20 | 3 | surg clip | Uncertain | 4 |
| 38 | F | 65 | I | 14 | 3 | pos (ACA) | 19 | 3 | surg clip ? VPS | Yes (Confusion) | 3 |
| 39 | M | 57 | II | 15 | 2 | neg | 4 | 1 | conservative | No | 5 |
| 40 | F | 55 | II | 15 | 1 | neg | 1 | 1 | conservative | No | 5 |
| 41 | M | 51 | II | 15 | 2 | neg | 5 | 1 | conservative | No | 5 |
| 42 | F | 60 | III | 13 | 3 | pos (MCA R) | 11 | 2 | surg clip | Uncertain | 4 |
neg=negative, pos=positive, ACA=anterior communicating artery, PCA=posterior communicating artery, MCA R(L)=right (left) middle cerebral artery, BA=basilar artery, IC=internal carotid, surg=surgical, endov=endovascular, (r) = followed by, VPS=venticulo-peritoneal shunt.
Clinical and laboratory investigation
Patients had a routine laboratory work-up, which included repeated blood tests and CT examinations. The amount of blood in the subarachnoid space was graded according to the Fisher scale. Patients´ clinical condition on admission was graded according to the Hunt and Hess scale (H&H) and the Glasgow Coma Score (GCS) (Table 1). Neurological status was carefully assessed daily during hospitalization. Digital subtraction arteriography was performed within the first 2 days from admission for the majority of patients, or later (after day 14), if a patient´s clinical condition did not permit. If DSA failed to reveal an aneurysm, the second DSA was performed 1-2 days before hospital discharge. Surgical clipping of the aneurysm demonstrated by the first or by the second DSA was a standard therapeutic procedure (Table 1). In 4 occasions endovascular treatment (coiling) was undertaken. Vasospasm was assumed on clinical basis if deterioration of consciousness, increase in body temperature or new neurological deficit developed at least 3 days after SAH that could not be explained by electrolyte or metabolic disturbances, hydrocephalus or re-bleeding. When a new neurological deficit occurred after surgery, it was attributed to vasospasm only if it was detected 48 hours or more after craniotomy, and if it could not be explained otherwise. The patients developing severe hydrocephalus acutely after SAH or post-operatively were treated with ventricular-peritoneal shunting. The outcome was assessed at 6 months after SAH and was graded according to the Glasgow outcome scale (GOS) (Table 1).
SPECT perfusion imaging
The SPECT studies were performed within the first 5 days from admission (mean 3.4 days, median 3 days). Attention was given to perform SPECT studies either before DSA, or 48 hours after DSA, in order to avoid possible blood flow disturbances induced by angiography. 99mTc-ECD (Neurolite(r), Bristol-Myers Squibb Pharm, Belgium) was reconstituted from a lyophilized commercial kit, according to the manufacturer´s instructions. Seven hundred and forty MBq of the radiopharmaceutical were administered through an intravenous line previously inserted. A patient was placed in a quiet dimly-lit room 10 minutes prior to injection and was instructed to remain calm, not to speak or read, to keep eyes open, until injection time and for the next 30 minutes thereafter. Imaging took place approximately 45 minutes post injection. A dual-head gamma camera (Helix, El Scint, Israel) was used, equipped with a parallel-hole, low energy, high-resolution collimator. A specific head-holder, provided by the manufacturer, was added to the imaging table in order to prevent movement and to minimize the distance between the heads of the camera and a patient´s head. Acquisition parameters were as follows: 120 projections (60 for each head) over an angular range of 360°, a step and shoot mode, 30 seconds per step, a body contour orbit, 128x128 matrix size, zoom 1.5. Raw images were pre-filtered with a Butterworth filter, order 10, critical frequency 0.5, and subsequent tomographic back-projection reconstruction was accomplished by ramp filtering. Attenuation correction with Chang´s method was applied to the tomographic slices, with a coefficient of 0.11 cm-1. Transverse, sagittal and coronal slices were re-oriented to the orbitomeatal axis. Transverse slice thickness was approximately 0.4 cm.
Quantification of SPECT analyses
Quantitative expression of SPECT findings was accomplished by semi-automated computer software. Counts over cerebral regions were normalized to the cerebellar maximum activity. A number of regions of interest (ROIs), mirrored around the midline, were automatically drawn on 6 manually selected transverse slices to encompass cortical and sub-cortical structures, as follows: Two ROIs over the cerebellum, 1 over the brain stem, 4 over the basal ganglia, 4 over the temporal cortex (medial and lateral) and another 48 ROIs over the cerebral cortex. The correct position of the automatic ROIs was manually re-adjusted. The right to left ratio, as well as the percentage of counts relative to the cerebellum were calculated for each region. A left to right ratio smaller than 0.95 or greater than 1.05 was considered abnormal. A relative percentage smaller than 60% of cerebellar counts was similarly considered abnormal, with the exception of medial occipital cortex and basal ganglia, where the cut-off of abnormal finding was set at 80% and 50% respectively. Summing of ROIs with abnormal findings yielded a final score of ischaemia Summed Perfusion Defect Score (SPDS) for each SPECT study (Table 1).
Statistics
Using the aforementioned criteria, ROC analysis of data derived from the database of our institution has shown that a SPDS cut-off of 9 could best discriminate abnormal subjects (those with neurological deficit or with GCS<15) from controls with 95% sensitivity, 95% specificity and 95% accuracy. SPECT findings of the present study were further graded as grade 1=normal (SPDS<9), grade 2=mildly-moderately abnormal (9ŁSPDSŁ16) and grade 3=severely abnormal (SPDS>16).
The correlation between sets of numerical and ordinal data, such as SPDS and H&H grade, or GCS, or Fisher scale, or GOS was evaluated by the rank correlation (Spearman´s rank regression) method. Differences of SPDS among different patient groups (e.g. those with or without vasospasm, and those with or without favourable outcome) were tested with the Mann-Whitney U test. The univariate analysis of the correlation between various risk factors with the development of vasospasm and with the outcome was accomplished by the c2 test (Yates correction). On this occasion, all variables were expressed in a dichotomous manner (e.g. low/high H&H grade, positive/negative SPECT, yes/no vasospasm, favourable/unfavourable outcome, etc).
In all analyses, p <0.05 was considered significant. Continuous variables are presented as mean ± SD.
Results
There were 42 patients who constituted the population of the study.
Patients´ characteristics, SPECT findings, clinical course and outcome are summarized in Table 1. The majority of patients included in the study were in a relatively good clinical condition on admission. The distribution of H&H grades were: H&H I, 6 patients; H&H II, 30 patients; H&H III, 6 patients. The distribution of GCS on admission were: GCS 15, 16 patients; GCS 14, 21 patients; GCS 13, 4 patients; GCS 12, 1 patient. Similarly, the majority of patients had CT scans of Fisher grade 2 (22 patients), whereas there was 1 patient with grade 1, another 14 with grade 3 and 5 patients with Fisher grade 4. DSA revealed an aneurysm in 20 patients, was negative in 21 cases, while it was not performed in one case. The most common sites of the aneurysm were the anterior communicating artery (10 cases) and the middle cerebral artery (7 cases), right (5 cases) or left (2 cases). Nineteen patients were treated with surgical clipping or endovascular coiling of the aneurysm, 8 with ventricular-peritoneal shunt (4 for postoperative hydrocephalus) and 19 conservatively.
Fifteen SPECT studies were within normal limits (SPECT grade 1). Among abnormal studies, 19 were of grade 2 and 8 of grade 3. SPDS was found to be associated with Fisher grade (Spearman´s coeff = 0.56, p = 0.0001), weakly with GCS on admission (Spearman´s coeff = -0.35, p = 0.02), but not with H&H grade (Spearman´s coeff = 0.08, p = ns). SPECT findings (SPDS or SPECT grade) did not correlate with patients´ age (Spearman´s coeff = 0.1, p = ns) or sex (c2= 0.02, p = ns) (Table 2). Thirteen of 21 patients (62%) with a non-aneurysmal DSA, opposed to 2 of 20 (10%) with aneurysm-positive DSA, had normal SPECT studies (c2= 11.9, p=0.001) (Table 2).
Table 2. Univariate correlation between various baseline variables and the development of vasospasm. The positive and negative predictive values of each variable are also compared.
| | CLINICAL VASOSPASM |
| Variable | x2 | p | Negative Predictive Value | Positive Predictive Value |
| SPECT (neg/pos) | 18.8 | 0.00001 | 1.00 | 0.75 |
| DSA (neg/pos*) | 7.4 | 0.01 | 0.78 | 0.69 |
| H&H (grade I-II/grade III) | 1.8 | ns | 0.60 | 0.75 |
| CT FISHER (grade 1-2/3-4) | 7.2 | 0.01 | 0.73 | 0.75 |
| GCS on admission (15/<15) | 7.0 | 0.01 | 0.85 | 0.62 |
| Age (<50/>=50) | 0.6 | ns | 0.67 | 0.48 |
| Sex (M/F) | 0.002 | ns | 0.56 | 0.44 |
* Positive or negative for the demonstration of an aneurysm; ns = not statistically significant (p>0.05).
Fifteen patients developed clinical vasospasm (Table 1). In six of these patients the vasospasm occurred postoperatively. The remaining patients with vasospasm either had been treated conservatively (4 cases), or by endovascular coiling (2 cases), or had developed vasospasm preoperatively (3 cases). In another 8 cases, vasospasm could not be excluded, but the diagnosis was uncertain, either because symptoms were mild or due to the presence of confounding factors, such as severe hydrocephalus or a very recent surgery. These 8 patients were excluded from the evaluation of vasospasm. An abnormal SPECT study was found in all 15 patients who developed vasospasm but also in 5 out of 19 who did not. Extensive perfusion defects (grade 3 SPECT) were observed in 4 patients who all subsequently presented vasospasm and in another 4 patients with possible, but unconfirmed, vasospasm. The range, mean ± SD and median values of SPDS were 1-14, 6.3 ± 4.1 and 5.0 respectively in the group without vasospasm, whereas the corresponding values in those who presented vasospasm were 11-19, 14.7 ± 2.5 and 14.0 respectively. The difference was statistically significant (p<0.001) and is schematically shown in (Figure 1). Among various possible risk factors, SPECT grade showed the closest correlation with the development of clinical vasospasm, as expressed by the magnitude of the c2 value (Table 2). In Table 2, the positive and negative predictive values of these risk factors are also shown, compared with those of SPECT. A negative SPECT study showed the highest negative predictive value (100%) for the occurrence of vasospasm, whereas the positive predictive value of SPECT (75%) was comparable with that of an H&H grade III and a CT Fisher grade of 3 or 4.
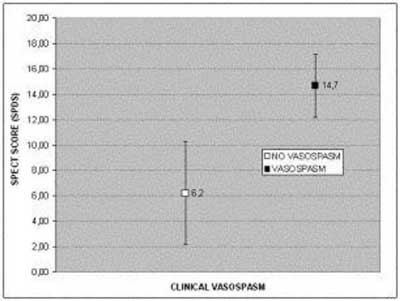
Figure 1. Comparison of SPDS between groups of patients with and without subsequent presentation of clinical vasospasm.
Nine patients died, either during their hospitalization (n = 3) or during the period of the follow-up (n = 6). The outcome of the remaining patients 6 months after SAH was: GOS 3, 6 patients; GOS 4, 8 patients; GOS 5, 19 patients. A statistically significant difference of the SPECT score (p = 0.003) was found between patient groups with favourable (GOS 4 or 5) and unfavourable outcome (GOS 1-3). The range, mean ± SD, and median values of SPDS in these two groups were 1-21, 9.0 ± 5.8, 7.0 and 7-22, 14.5 ± 4.1, 15.0 respectively. Regression analysis between SPDS values and GOS grade showed a weak, but statistically significant, correlation between these two variables (Spearman´s coeff = -0.47, p = 0.002). This weak correlation was due to poor discrimination of GOS 1, GOS 3 and GOS 4 groups between each other, whereas the group with GOS 5 differed significantly from all other groups (Mann-Whitney U test, p<0.01), as it is schematically shown in Figure 2. A normal baseline SPECT study was observed in 68.4% (13/19) of the patients with GOS 5 and in 51.9% (14/27) of the patients with GOS 4 or 5. On the other hand, 93.3% (14/15) of the patients with a GOS grade 1-3 had abnormal SPECT studies. Extensive perfusion abnormalities (SPECT grade 3) were observed early in the course of SAH in 3 patients with a good outcome (GOS 4 or 5) and in 5 patients with an unfavourable outcome. In Table 3, the results of univariate analysis between various predictor variables and the clinical outcome are compared. Age and initial CT findings (Fisher grade) showed the closest correlation, followed by SPECT and the occurrence of symptomatic vasospasm. Among various risk factors, SPECT had the highest negative predictive value (Table 3), while its positive predictive value for an unfavourable outcome was low.
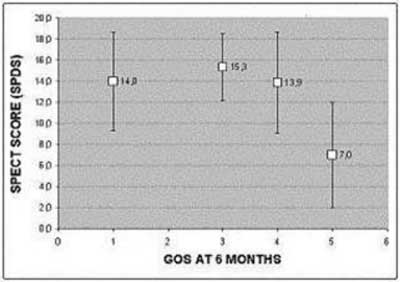
Figure 2. SPDS plotted against GOS grade, assessed 6 months after the onset of SAH.
Table 3. Univariate correlation between various predictor variables and the 6-month outcome. All variables are expressed in a dichotomous manner. Clinical outcome, the response variable, is expressed as satisfactory (GOS 4-5) or unsatisfactory (GOS 1-3). The positive and negative predictive values of each predictor variable are also compared.
| | OUTCOME (GOS) |
| Variable | c2 | p | Negative Predictive Value | Positive Predictive Value |
| SPECT (neg/pos*) | 8.6 | 0.003 | 0.93 | 0.52 |
| DSA (neg/pos*) | 4.4 | 0.04 | 0.81 | 0.50 |
| H&H (grade I-II/grade III) | 0.02 | ns | 0.64 | 0.33 |
| CT FISHER (grade 1-2/3-4) | 11.4 | 0.001 | 0.87 | 0.63 |
| GCS on admission (15/<15) | 0.2 | ns | 0.69 | 0.38 |
| Vasospasm (no/yes) | 7.2 | 0.01 | 0.84 | 0.60 |
| Surgical treatment (no/yes) | 1.6 | ns | 0.72 | 0.47 |
| Age (<50/>=50) | 12.6 | 0.03 | 0.91 | 0.45 |
| Sex (M/F) | 0.7 | ns | 0.65 | 0.47 |
* Positive or negative for the demonstration of an aneurysm; ns=not statistically significant (p>0.05).
Discussion
Cerebral vasospasm remains a devastating medical complication of SAH, leaving about one third of its victims with permanent ischaemic neurological deficits. Vasospasm is a result of blood in the subarachnoid space. Blood products and lipid peroxidation by oxygen free radicals are thought to play an important role in both arterial spasm and ischemic cell death. Some authors have claimed that a close relationship exists between the location of subarachnoid blood and the thickness of the blood clot on one hand, and the occurrence of vasospasm and delayed cerebral ischaemia on the other (32). However, other factors probably participate. Secondary ischaemia does not occur in non-aneurysmal SAH and is rare in patients with SAH secondary to intracerebral haematoma or a ruptured arteriovenous malformation (1). The probability of occurrence of symptomatic vasospasm is also probably associated with age, neurological grade measured on admission and hyperglycemia (4).
Diagnosis of vasospasm remains difficult. Conventional angiography was considered a reference technique, but carries a risk of complications and gives no information on tissue perfusion. Transcranial Doppler, a noninvasive method, gives indirect evidence of vasospasm but the consequences of vasospasm on the brain parenchyma are also not shown. Moreover, some patients remain asymptomatic despite severe vasospasm, while others present with neurological deficits even at only moderate blood flow velocity increases. Early diagnosis of clinically significant cerebral vasospasm is of prime importance, because therapeutic actions, either medical or interventional, are expected to be efficacious only before a cerebral infarct has been launched. Furthermore, an early, accurate, risk stratification algorithm would enable the implementation of a rigorous preventive therapy to high-risk patients, while inversely those with low-risk would be deferred from unnecessary and potentially harmful medication.
Brain perfusion SPECT has been utilized in order to directly demonstrate regional cerebral ischemia secondary to SAH. Powsner et al, have reported 89% sensitivity and 71% specificity of SPECT for the detection of vasospasm. Other investigators, using a quantitative SPECT method, have quoted similar figures (27). However, regional hypoperfusion seen on SPECT is not always due to vasospasm, even if other apparent causes such as prior neurological or psychiatric diseases have been excluded from the history. Hydrocephalus can also produce areas of hypoperfusion, mainly in the basal parts of the brain (19). Post-operative perfusion defects due to cerebral edema, haematoma or brain contraction are common, as has been stated by certain authors, and should not be misinterpreted as vasospasm (21, 23, 27, 29). Consequently, in view of the current tendency for early surgery in order to avoid rebleeding, the usefulness of SPECT in a number of patients who are suspected of postoperative vasospasm is questionable, at least during the first week after surgery (27). On the contrary, in a group of patients treated with endovascular coiling, Koivisto et al, did not observe deterioration of perfusion defects after treatment, compared with the baseline SPECT study (29).
In the present study we investigated the predictive value of SPECT performed within the first few days from SAH, before surgical or endovascular treatment is undertaken and before vasospasm occurs, or in its very early stages. The majority of patients had abnormal SPECT studies (27/42, 63.2%), a finding, which is in accordance with previous reports (16, 18, 29, 30). It seems that regional disturbances of blood flow are common, early after SAH. Some of these perfusion defects are of no clinical importance, and probably some resolve in due course, since a number of patients have an uncomplicated course despite initial regional hypoperfusion (5 patients in the present study). However, in the present study, the majority of patients with abnormal perfusion either developed vasospasm (15/27, 55.5%), or had clinical deterioration possibly related to vasospasm or possibly further complicated by vasospasm (7/27, 25.9%). The positive predictive value of SPECT for the occurrence of vasospasm was 75%, comparable with that of other risk factors, such as the amount of subarachnoid blood seen on CT. In agreement with our results, Biroli et al, have reported early blood flow diminution detected by SPECT in patients who subsequently presented clinical deterioration (16). Similarly, Ohkuma et al, found that areas of patchy decreased perfusion seen on SPECT preceded the onset of delayed ischaemic neurological deficit (30). By the use of acetazolamide activated SPECT, a group of investigators were able to detect early changes in vasodilatory capacity, which predicted cerebral infarction due to vasospasm (17, 18). On the other hand, a normal baseline SPECT seems highly predictive of a favourable clinical course. In the present study, all 15 patients with a normal SPECT had an uncomplicated course during hospitalization (negative predictive value of SPECT 100%). Among various probable risk factors, a negative SPECT study exhibited the highest negative predictive value for subsequent symptomatic vasospasm (Table 2).
All patients with a normal baseline SPECT exited hospital with a GOS grade of 4 or 5 (negative predictive value for a satisfactory short-term outcome 100%). A low Fisher grade and a non-aneurismal DSA were found, expectedly, to carry also a high negative predictive value (95% and 91% respectively). On the contrary, the positive predictive value of SPECT was low (40.7%). Even extensive baseline blood flow abnormalities (SPECT grade 3), predicted an unsatisfactory short-term outcome in only 50% of patients. Resolution of initial perfusion defects or clinically insignificant blood flow abnormalities is an apparent explanation of this finding. Predictably, the occurrence of symptomatic vasospasm exhibited the highest positive predictive value (67%) for an adverse short-term outcome, and was also found to have a 100% negative predictive value.
When GOS was assessed 6 months after the onset of SAH, the negative predictive value of SPECT was also high (93.3%), higher than any other risk factor, while its positive predictive value was 52%. In this instance, the amount of blood in the subarachnoid space on the baseline CT examination and the occurrence of vasospasm were found to be better predictors of an unsatisfactory mid-term outcome (Table 3).
Conclusion – Clinical implications
The results of the present study suggest that the use of brain perfusion SPECT within the first few days after hospital admission for SAH can provide important prognostic information. A normal SPECT study appears highly predictive of an uncomplicated clinical course and of a favourable short and mid-term outcome. Regional perfusion abnormalities are common, early after SAH. Its positive predictive value for subsequent symptomatic vasospasm is probably comparable with that of other risk factors, such as the amount of blood in the subarachnoid space. However, regarding the clinical outcome, the positive predictive value of early blood flow disturbances is poor.
Finally, the clinical implication of the SPECT-ECD imaging, calculating the SPDS needs to be further evaluated in a large number of patients.
Acknowledgement
The authors thank the medical statistician Dr. Alexia Zaganides for her assistance.
Piśmiennictwo
1. Van Gijn J, Rinkel GJE: Subarachnoid Hemorrhage: Diagnosis, causes and management. Brain 2001: 124:249-278. 2.Ohman J, Heiskanen O.: Timing of operation for ruptured supratentorial aneurysms: a prospective randomized study. J Neurosurgery 1989: 70:55-60. 3.Hijdra A, van Gijn J, Stefanko S, et al.: Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: clinicoanatomic correlations. Neurology 1986: 36:329-333. 4.Charpentier C, Audibert G, Guillemin F, et al.: Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 1999: 30:1402-1408. 5.Kassel NF, Peerless SJ, Durward QJ.: Treatment of ischaemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery 1982: 11:337-343. 6.Barker FG, Ogilvy CS.: Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a meta analysis. J Neurosurg 1996: 84:405-414. 7.Sen J, Belli A, Albon H, et al.: Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol 2003: 2: 614-621. 8.Elliot JP, Newell DW, Lam DJ, et al.: Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 1998: 88:277-284. 9.Cloft HJ, Joseph GJ, Dion JE.: Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999: 30:317-320. 10.Mascia L, Fedorko L, Brugge K, et al.: The accuracy of transcranial Doppler to detect vasospasm in patients with aneurysmal subarachnoid haemorrhage. Intensive Care Med 2003: 29:1088-1094. 11. Grandin CB, Cosnard G, Hammer F, et al.: Vasospasm after Subarachnoid Hemorrhage: Diagnosis with MR Angiography. AJNR Am J Neuroradiol 2000: 21:1611-1617. 12.Preda L, Gaetani P, Rodriguez Y, et al.: Spiral CT angiography and surgical correlations in the evaluation of intracranial aneurysms. Eur Radiol 1998: 8:739-745. 13.Clyde BL, Resnick DK, Yonas H, et al.: The relationship in blood velocity as measured by transcranial Doppler ultrasonography to cerebral blood flow as determined by stable xenon computed tomographic studies after aneurysmal subarachnoid hemorrhage. Neurosurgery 1996: 38:896-905. 14.Condette-Auliac S, Bracard S, Anxionnat R, et al.: Vasospasm After Subarachnoid Hemorrhage. Interest in Diffusion-Weighted MR Imaging. Stroke 2001: 32:1818-1824. 15.Powers WJ, Grubb RL, Baker RP, et al.: Regional cerebral blood flow and metabolism in reversible ischemia due to vasospasm. Determination of positron emission tomography. J Neurosurg 1985: 4:539. 16.Biroli F, Ferraresi S, Degonda F, et al.: Single-photon emission-computed tomography (SPECT) during the first 72 hours after subarachnoid hemorrhage: a study of 45 cases. Agressologie 1990: 31:257-258. 17.Shinoda J, Kimura T, Funakoshi T, et al.: Acetazolamide reactivity on cerebral blood flow in patients with subarachnoid haemorrhage. Acta Neurochir (Wien) 1991: 109:102-108. 18.Kimura T, Shinoda J, Funakoshi T.: Prediction of cerebral infarction due to vasospasm following aneurysmal subarachnoid haemorrhage using acetazolamide-activated 123I-IMP SPECT. Acta Neurochir (Wien) 1993: 123:125-128. 19.Hasan D, van Peski J, Loeve I, et al.: Single photon emission computed tomography in patients with acute hydrocephalus or with cerebral ischaemia after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1991: 54:490-493. 20.Lewis DH, Eskridge JM, Newell DW, et al.: Brain SPECT and the effect of cerebral angioplasty in delayed ischemia due to vasospasm. J Nucl Med 1992: 33:1789-1796. 21.Tranquart F, Ades PE, Groussin P, et al.: Postoperative assessment of cerebral blood flow in subarachnoid haemorrhage by means of 99mTc-HMPAO tomography. Eur J Nucl Med 1993: 20:53-58. 22.Naderi S, Ozguven MA, Bayhan H, et al.: Evaluation of cerebral vasospasm in patients with subarachnoid hemorrhage using single photon emission computed tomography. Neurosurg Rev 1994: 17:261-265. 23.Rosen JM, Butala AV, Oropello JM, et al.: Postoperative changes on brain SPECT imaging after aneurysmal subarachnoid haemorrhage. A potential pitfall in the evaluation of vasospasm. Clin Nucl Med 1994: 19:595-597. 24. Lewis DH, Newell DW, Winn HR.: Delayed ischemia due to cerebral vasospasm occult to transcranial Doppler. An important role for cerebral perfusion SPECT. Clin Nucl Med 1997: 22:238-240. 25.Powsner RA, O´Tuama LA, Jabre A, et al.: SPECT imaging in cerebral vasospasm following subarachnoid hemorrhage. J Nucl Med 1998: 39:765-769. 26.Rajendran JG, Lewis DH, Newell DW, et al.: Brain SPECT used to evaluate vasospasm after subarachnoid hemorrhage: correlation with angiography and transcranial Doppler. Clin Nucl Med 2001: 26:125-130. 27.Hosono M, Machida K, Matsui T, et al.: Non-invasive quantitative monitoring of cerebral blood flow in aneurysmal subarachnoid haemorrhage with 99mTc-ECD. Nucl Med Commun 2002: 23:5-11. 28. Jabre A, Babikian V, Powsner RA, et al.: Role of single photon emission computed tomography and transcranial Doppler ultrasonography in clinical vasospasm. Journal of Clinical Neuroscience 2002: 9:400-403. 29.Koivisto T, Vanninen E, Vanninen R, et al.: Cerebral perfusion before and after endovascular or surgical treatment of acutely ruptured cerebral aneurysms: a 1-year prospective follow-up study. Neurosurgery 2002: 51:312-225. 30.Ohkuma H, Suzuki S, Kudo K, et al.: Cortical blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage: three-dimensional N-isopropyl-p- [(123) I] iodoamphetamine single photon emission CT findings. AJNR Am J Neuroradiol 2003: 24:444-450. 31.Sviri GE, Lewis DH, Correa R, et al.: Basilar Artery Vasospasm and Delayed Posterior Circulation Ischemia After Aneurysmal Subarachnoid Haemorrhage. Stroke 2004: 35:1867-1872. 32.Kistler JP, Crowell RM, Davis KR, et al.: The relation of cerebral vasospasm to the extent and location of subarachnoid blood visualized by CT scan: a prospective study. Neurology 1983: 33:424-436.

