Zoltán Szakács1, *Terèzia Seres2, Éva Kellős2, Márta Simon1, Attila Terray Horváth1, Veronika Fáy3, Jelena Karaszova3, Andrea Kontra3, Olívia Lalátka3, Mária Csóka4, Gyula Domján5
Prevalence of rem behavioral disorder and rem sleep without atonia in patients suffering from parkinson’s disease
1Department of Neurology, Medical Centre, Hungarian Defence Forces, Budapest, Hungary
Head of Department: Zoltán Szakács, MD, PhD
2Doctoral School, Semmelweis University, Budapest, Hungary
Head of Department: Gyula Domján, MD, PhD
3Rehabilitation Centre, Combined Szent István and Szent László Hospital, Budapest, Hungary
Head of Centre: Veronika Fáy, MD, PhD
4Department of Nursing, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
Head of Department: Zoltán Balogh, MD, PhD
5Department of Clinical Studies, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
Head of Department: Klára Gadó, MD, PhD
Summary
Introduction. The atypical, non-motor symptoms of Parkinson’s disease have been drawing more and more attention recently. These symptoms include: neuropsychiatric dysfunctions, dysautonomy, sleep disorders and sensory symptoms, such as pain. Neurodegeneration resulting from Parkinson’s disease may affect the REM-on and REM-off neurons that are responsible for the structure of sleep. This may result in sleep fragmentation, decreased sleep efficiency, decreased amount of deep sleep and REM sleep and behavioral disorders during REM sleep (REM sleep behavior disorder – RBD). RBD is a primary sleep disorder that is characterized by the appearance of the activity of skeletal muscles during REM sleep. REM sleep without atonia (RWA), on the other hand, is characterized by abnormal muscle activation without complex behavioral expression.
Aim. To determine the prevalence of REM sleep without atonia and RBD in patients with Parkinson's disease.
Material and methods. We assessed the frequency of RWA and RDB in 50 non-selected patients with Parkinson’s disease by a polisomnographic observation (PSG). A demographic analysis was conducted. 50 patients, aged on average 71.9 ± 11.8 years and suffering from Parkinson’s disease, average Hoehn-Yahr stadium 1.9 ± 0.8, participated in the study. The results were compared to the data of 16 healthy control persons without sleep disorders with age and gender matching the study group (average age 62.31 ± 6.87 years).
When RWA is suspected in a patient, additional monitoring during polysomnography must be ensured. Apart from the obligatory video monitoring, EMG channels recording the tone on all the four limbs (musculi tibialis anterior, soleus and biceps brachii bilaterally) and in the chin muscle are recommended.
Results. RBD was present in the anamnesis of 4 (8%) patients with Parkinson’s disease. During polysomnography, RWA was detected in 17 patients (34%). In the vast majority of cases, no behavioral manifestation of RBD could be detected. RBD patients were characterized by a much higher limb movement index in the REM phase (18.6 ± 4.39 events/hour) than the control group (4.4 ± 2.3 events/hour; p = 0.0001). During their sleep, RBD patients spent more time in the deep slow-wave sleep (2.64 ± 1.31%) than the control group (0.76 ± 0.27%; p = 0.004). Furthermore, the RBD patients had a higher percentage of REM sleep (12.8 ± 3.19% vs. 8.6 ± 1.67%; p = 0.01) than the controls. REM density was lower in RBD patients than in the control group (20.8 ± 2.77% vs. 31.2 ± 4.16%; p = 0.01). During their REM sleep, patients with Parkinson’s disease spent lower amount of time in muscle atonia than the persons from the control group (61.5 vs. 95.6%; p = 0.004).
Conclusions. RWA was significantly more prevalent among patients with Parkinson’s disease than in the healthy persons. Furthermore, nearly two-thirds of the patients suffering from Parkinson’s disease had submental tonic muscle activity detected in EMG in at least 20% of their total REM sleep duration. REM sleep without atonia was detected in many patients with Parkinson’s disease without anamnestic data suggesting sleep disorder or behavioral disorder during REM sleep. The connection between RBD and RWA is still unclear, however, two mutually non-exclusive hypotheses can provide an explanation for the phenomenon of RBD without RWA. Longitudinal studies with PSG examinations are needed to clarify whether, with the passage of time, the clinical features of RBD indeed evolve to RWA in patients without behavior disorder.
Introduction
The atypical, non-motor symptoms of Parkinson’s disease have been drawing more and more attention recently. These symptoms include: neuropsychiatric dysfunctions, dysautonomy, sleep disorders and sensory symptoms, such as pain (1). Neurodegeneration resulting from Parkinson’s disease may affect the REM-on and REM-off neurons that are responsible for the sleep structure. This may result in sleep fragmentation, decreased sleep efficiency, decreased amount of deep sleep and REM sleep and behavioral disorders during the REM sleep (REM Sleep Behavior Disorder – RBD) (2-4).
RBD is a primary sleep disorder that is characterized by the appearance of the activity of skeletal muscles during REM sleep (5).
REM sleep without atonia (RWA), on the other hand, is characterized by abnormal muscle activation without complex behavioral expression. In the polysomnography findings of patients with RBD, chin muscle activity and limb movement and can often be detected in the REM sleep. Complex and often violent behavior may be present. RBD can frequently cause skin injuries and fractures to the patients and their sleeping partners. Although the clinical symptoms of RBD are rather specific to the disease, other primary sleep disorders, such as sleep apnea, can manifest similarly. REM sleep appears approximately every 90-120 minutes during sleep, therefore, RBD episodes happen up to four times a night. Rarely can they appear only once in a week or even once in a month (6-7).
Polysomnographic test is needed in order to confirm the diagnosis of RBD. 25-50% of the patients with Parkinson’s disease additionally suffer from RBD or RWA. The prevalence of these disorders is even higher in patients with multiple system atrophy (MSA) and dementia with Lewy bodies (DLB). RBD often appears years before the onset of the symptoms of Parkinsonism. In the early stage of Parkinson’s disease, extensive pathological changes occur in the REM-sleep controlling areas in the brain stem – the very same areas that are involved in the pathophysiology of parkinsonism (8). These observations led to the hypothesis that RBD is an early stage of synucleinopathies, i.e. Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy (9-14).
RBD was firstly described as a separate condition in 1986 (15). RBD can be described as a loss of atonia of skeletal muscles, which normally appears during the REM sleep, and by the complex motor activity while dreaming. According to the International Classification of Sleep Disorder (ICSD), the minimal diagnostic criteria for RBD include limb or body movements related to dreams and at least one of the followings: harmful or potentially harmful sleep behavior; acting out of the dreams; sleep behavior that breaks the continuity of sleep (16).
Most of the patients (87-90%) suffering from RBD are elderly men. The patients may cause severe injuries to themselves as well as to their sleeping partners (17, 18). RBD is often associated neurodegenerative diseases (19-21) from the group of synucleinopathies (Parkinson’s disease, MSA dementia with Lewy bodies) (18, 22, 23). In several case reports, the symptoms of RBD appeared 3-13 years earlier than the symptoms of Parkinson’s disease (18, 22). In spite of this, only few studied the association of RBD and Parkinson’s disease and none of them used PSG recordings and or assessed the sleep complaints of patients suffering from Parkinson’s disease.
Aim
Sleep disorders have a high prevalence in patients with Parkinson's disease. Together with the underlying motor symptoms, sleep disorders are the main causes of disability and have a substantial impact on the quality of life of these patients. Of particular interest are the behavior disorders of REM sleep (RBD) which are reported in many cases to precede the development of Parkinson's disease. Our aim was to determine the frequency of REM sleep behavior disorder among patients with PD using polysomnography recordings.
Material and methods
Patients
We assessed the frequency of RWA and RDB in 50 non-selected patients with Parkinson’s disease by a polysomnographic observation (PSG). A demographic analysis was conducted. 50 patients, aged on average 71.9 ± 11.8 years and with average Hoehn-Yahr stadium 1.9 ± 0.8, participated in the study. The results were compared with the data of 16 healthy controls without sleep disorders with age and gender matching the study group (average age 62.31 ± 6.87 years).
When RWA is suspected in a patient, additional monitoring during polysomnography must be ensured. Apart from the obligatory video monitoring, EMG channels recording the tone on all the four limbs (musculi tibialis anterior, soleus and biceps brachii bilaterally) and in the chin muscle are recommended.
Each patient spent one night in the sleep laboratory. We recruited the patients from the Neurology Department of the Health Centre of the Hungarian Army in Budapest. The patients were enrolled in the study regardless of their sleeping complaints and were asked to participate in it during their checkup visits. Only the patients with idiopathic Parkinson’s disease, Hoehn-Yahr stadium from 1 to 3, could take part in the study. Each patient had bradykinesia and at least one of the typical symptoms of the Parkinson’s disease: resting tremor and rigidity or impairment of the postural reflexes. Patients with atypical symptoms, such as pyramidal or cerebellar symptoms signs and symptoms, oculomotor palsy, dyspraxia or autonomic dysfunction, were excluded from the study. Patients fulfilling the DSM-V criteria for depression and dementia were also excluded. Neither the patients nor their relatives reported visual hallucinations or fluctuating cognition, which are regarded to be typical signs of the dementia with Lewy bodies. The anamnesis of none of the patients revealed an exposition to toxic materials, head injury, encephalitis or cerebrovascular disease.
44 patients were treated with dopaminergic medications: 39 with levodopa (average daily dosage: 510 mg); 13 with selegiline (average daily dosage: 5 mg); 12 with pramipexole (average daily dosage: 4.3 mg); 3 with bromocriptine (average daily dosage: 18 mg); 2 with amantadine (average daily dosage: 150 mg) and 2 with ropinirole (average daily dosage: 14 mg). One patient had not been taking any medications for 3 months and 5 patients had not undergone any treatment for Parkinson’s disease yet.
None of the participants, either from the study group or the control groups, had been taking benzodiazepines, tricyclic antidepressants or SSRIs. Furthermore, the persons from the control group did not use any medication that could influence sleep or motor activity. The study was approved by the ethical committee of the Hospital.
Polysomnographic recordings
The lights were extinguished at the regular time of getting into bed of the patient (between 21:42 and 23:29). During the recording, data from the following diversions was collected to define sleeping stages: right and left side electrooculogram (EOG), submental EMG and two-two centralis (C3-A2, C4-A2) as well as occipitalis (O1-A2, O2-A1) EEG diversions. When RWA is suspected in a patient, additional monitoring during polysomnography must be ensured. Apart from the obligatory video monitoring, EMG channels recording the tone on all the four limbs (musculi tibialis anterior, soleus and biceps brachii bilaterally) and in the chin muscle are recommended. We also used an infrared video recorder to detect patients’ movements during REM sleep. To define REM sleep stage, we used exclusively EEG and electrooculogram. Latency, defined as the interval from the sleep onset to the first appearance of REM sleep, was also measured. The end of the REM sleep period was indicated on the grounds of specific EEG graphoelements indicating other sleep stages (K-complexes, sleep spindles or arousal spikes) as well as on the grounds of continuous lack of rapid eye movements longer than three minutes. We repeated the procedure in the control group. To define sleep stages from 1 to 4, epochs of 30 seconds width were used and modified Rechtschaffen and Kales scoring criteria was applied. The epochs were classified into the tonic or atonic epoch group according to the tonic EMG activity of the chin muscle. The epoch was categorized as tonic if there was tonic EMG activity measurable in at least 50% of its time and as atonic if tonic EMG activity lasted for less than 50% of the epoch. The cut-off for the tonic activity was set individually for each patient and amounted between 3 and 7uV. EMG sign was declared to be tonic if its amplitude was twice as high as the amplitude measured during atonia and higher than 10uV. We used shorter atonic periods to determine the cut-off at any given time. The values measured were influenced by the background noises from the recording system, from the impedance of the electrodes as well as the artifacts. The following PSG criteria were used to diagnose RBD: an increase of submental EMG muscle tone during REM sleep (in more than 20% of the total REM sleep time), accompanied by complex motor activities in the video recording (e.g. speaking, laughing, shouting, trepidation, gestures, packing, hitting, sitting up or kicking) simultaneous with PSG findings.
Results
RBD was present in the anamnesis of 4 (8%) patients with Parkinson’s disease. During polysomnography, RWA was detected in 17 patients (34%). In vast majority of cases, no behavioral manifestation of RBD could be detected.
RBD patients were characterized by a much higher limb movement index in the REM phase (18.6 ± 4.39 events/hour) than the control group (4.4 ± 2.3 events/hour; p = 0.0001) (fig. 1).
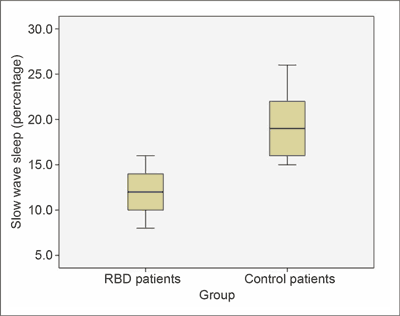
Fig. 1. In RBD patients, the limb movement index was much higher in REM phase than in the controls
During their sleep, RBD patients spent more time in the deep slow-wave sleep (2.64 ± 1.31%) than the control group (0.76 ± 0.27%; p = 0.004) (fig. 2).

Fig. 2. During their sleep, RBD patients spent more time in deep slow-wave sleep than controls
Furthermore, the RBD patients had a higher share of REM sleep in the total sleep time (12.8 ± 3.19% vs. 8.6 ± 1.67%; p = 0.01) (fig. 3) than the controls. REM density was lower in RBD patients than in the control group (20.8 ± 2.77% vs. 31.2 ± 4.16%; p = 0.01) (fig. 4).
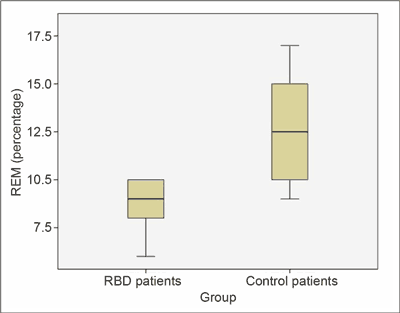
Fig. 3. RBD patients had a higher percentage of REM sleep in the total sleep time than controls
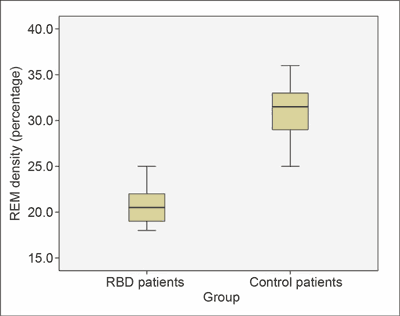
Fig. 4. REM density in RBD patients was lower than in controls
During their REM sleep, patients with Parkinson’s disease spent lower amounts of time in muscle atonia than the persons from the control group (61.5 vs. 95.6%; p = 0.004) (fig. 5).
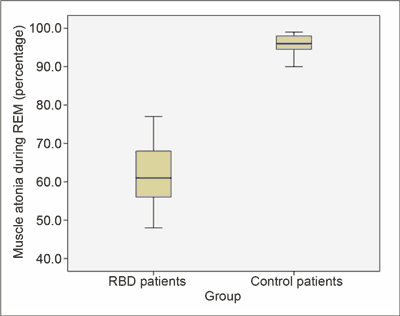
Fig. 5. During their REM sleep, patients with Parkinson’s disease spent lower amount of time in muscle atonia than controls
In our study, half of the RBD cases would have remained unrecognized if a structured clinical questionnaire had been the only tool used to make the diagnosis. The frequency of RBD detected by PSG was lower in our study than the frequency of RBD detected by other researchers during PSG examinations (17). However, it is worth noting that only patients with Parkinson’s disease and sleeping problems (including nocturnal complex motor activities) were included in the other studies (18). This criterion explains why a higher prevalence of RBD was detected in these studies. The detected gender proportion of RBD in Parkinson’s disease patients was similar to other findings for idiopathic RBD (1, 19, 20).
Discussion
According to the International Classification of Sleep Disorder (16), PSG examination is not required to establish RBD diagnosis. However, as our own results and the results of other researchers suggest, the detection rate of RBD in patients with Parkinson’s disease can be significantly increased when applying PSG (17, 25).
RWA was significantly more prevalent among patients with Parkinson’s disease than in the healthy controls. Furthermore, nearly two-thirds of our patients with Parkinson’s disease had submental tonic EMG activity in at least 20% of their total REM sleep period. REM sleep without atonia can be detected in several patients suffering from Parkinson’s disease without anamnestic data regarding sleep disorders and without behavior disorder during REM in the sleep laboratory. The connection between RBD and RWA is still unclear, however, two mutually non-exclusive hypotheses can provide an explanation for the phenomenon of RBD without RWA.
Longitudinal studies with PSG examinations are needed to clarify whether, with the passage of time, the clinical features of RBD indeed evolve to RWA in patients without behavior disorder.
The standard method based on the EMG muscle activity of the chin has been applied in the study to qualify the REM muscle atonia. The EMG signals routed from the musculus tibialis anterior are specific and sensitive for the detection of RBD. The loss of muscle atonia presumably reflects that degenerative process, which is responsible for the formation of RBD, although, due to its role in causing behavioral manifestations, an increase in the EMG activity might be an important factor as well.
Parkinson’s disease damages the REM sleep-moduling centers, as the disease affects lower brain stem much sooner than formulae of the bridge and substantia nigra (21-24). Locus coeruleus is also affected (25-27). Studies proved the impairment of locus coeruleus neurons and cholinergic neurons in Parkinson’s disease (28, 29). A 40% decrease in the number of cholinergic neurons, appearance of Lewy Bodies in the lower brain stem and in the pedunculopontine tegmental core indicate the influence of Parkinson’s disease on REM sleep (30, 31).
Conclusions
RWA was significantly more prevalent among patients with Parkinson’s disease than in the healthy persons. Furthermore, nearly two-thirds of the patients suffering from Parkinson’s disease had submental tonic muscle activity detected in EMG in at least 20% of their total REM sleep duration. REM sleep without atonia was detected in many patients with Parkinson’s disease without anamnestic data suggesting sleep disorder or behavioral disorder during REM sleep. The connection between RBD and RWA is still unclear, however, two mutually non-exclusive hypotheses can provide an explanation for the phenomenon of RBD without RWA. Longitudinal studies with PSG examinations are needed to clarify whether, with the passage of time, the clinical features of RBD indeed evolve to RWA in patients without behavior disorder.
Piśmiennictwo
1. Barone P, Antonini A, Colosimo C et al.: The Priamo study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Movement Disorders 2009; 24(11): 1641-1649. 2. Schenck C, Mahowald M: REM sleep behavior disorder: Clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep 2002; 25: 120-138. 3. Schenck C: Paradox Lost – Midnight in the Battleground of Sleep and Dreams – Violent Moving Nightmares, REM Sleep Behavior Disorder. Extreme-Nights, LLC 2005. 4. Boeve B, Silber M, Saper C et al.: Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007; 130: 2770-2788. 5. Gagnon JF, Medard MA, Fantini M et al.: REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology 2002; 59: 585-589. 6. Postuma RB, Gagnon JF, Rompre S, Montplaisir JY: Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology 2010 Jan 19; 74(3): 239-244. 7. Ferri R, Fulda S, Cosentino FI et al.: A preliminary quantitative analysis of REM sleep chin EMG in Parkinson’s disease with or without REM sleep behavior disorder. Sleep Med 2012 Jun; 13(6): 707-713. 8. de la Fuente-Fernandez R: A predictive model of neurodegeneration in idiopathic REM-sleep behavior disorder. Parkinsonism Relat Disord 2013; 19(11): 1009-1012. 9. Boeve BF, Silber MH, Ferman TJ et al.: REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 1998; 51: 363-370. 10. Turner RS: Idiopathic rapid eye movement sleep behavior disorder is a harbinger of dementia with Lewy bodies. J Geriatr Psychiatr Neurol 2002; 15: 195-199. 11. Iranzo A, Santamaria J, Rye DB et al.: Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD. Neurology 2005; 65: 247-252. 12. Iranzo A, Molinuevo J, Santamaría J et al.: Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006; 5: 572-577. 13. Boeve B, Silber M, Ferman T et al.: REM Sleep Behavior Disorder in Parkinson’s Disease, Dementia with Lewy Bodies, and Multiple System Atrophy. [In:] Bedard M, Agid Y, Chouinard S et al. (eds.): Mental and Behavioral Dysfunction in Movement Disorders. Humana Press, Totowa 2003: 383-397. 14. Claassen D, Josephs K, Ahlskog J et al.: REM sleep behavior disorder may precede PD, DLB, or MSA by up to half a century. Neurology 2009; 72 (suppl. 3): A324. 15. Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW: Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep 1986; 9: 293-308. 16. American Sleep Disorders Association: International classification of sleep disorders, revised: diagnostic and coding manual. American Sleep Disorders Association, Rochester, MN 1997: 177-180. 17. Eisensehr I, Lindeiner H, Jager M et al.: REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson’s disease: is there a need for polysomnography? J Neurol Sci 2001; 186: 7-11. 18. Gagnon J-F, Bedard M-A, Fantini ML et al.: REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology 2002; 59: 585-589. 19. Mahowald MW, Schenck CH: REM sleep parasomnias. [In:] Kryger MH, Roth T, Dement WC (eds.): Principles and practice of sleep medicine. 3rd ed. WB Saunders, Philadelphia 2000: 724-741. 20. Olson EJ, Boeve BF, Silber MH: Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain 2000; 123: 331-339. 21. Baba M, Nakajo S, Tu PH et al.: Aggregation of alphasynuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 1998; 152: 879-884. 22. Boeve BF, Silber MH, Ferman TJ et al.: Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord 2001; 16: 622-630. 23. Hardy J, Gwinn-Hardy K: Genetic classification of primary neurodegenerative disease. Science 1998; 282: 1075-1079. 24. Sforza E, Krieger J, Petiau C: REM sleep behavior disorder: clinical and physiopathological findings. Sleep Med Rev 1997; 1: 57-69. 25. Plazzi G, Corsini R, Provini F et al.: REM sleep behavior disorders in multiple system atrophy. Neurology 1997; 48: 1094-1097. 26. Boeve BF, Silber MH, Ferman TJ et al.: REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 1998; 51: 363-370. 27. Lapierre O, Montplaisir J: Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology 1992; 42: 1371-1374. 28. Hendricks J, Morrison A, Mann G: Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res 1982; 239: 81-105. 29. Jouvet M, Delorme F: Locus coeruleus et sommeil paradoxal. C R Soc Biol 1965; 159: 895-899. 30. Lai Y, Siegel J: Medullary regions mediating atonia. J Neurosci 1988; 8: 4790-4796. 31. Lai Y, Siegel J: Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci 1990; 10: 2727-2734.




