Agata Wasilewska1, Małgorzata Badełek-Izdebska1, *Lidia Zawadzka-Głos1, Remigiusz Krysiak2, Jarosław Żyłkowski3
Minimally invasive therapy of lymphatic malformations in patients treated in the Paediatric Teaching Clinical Hospital, University Clinical Centre of the Medical University of Warsaw (DSK UCK WUM)
Małoinwazyjne metody leczenia malformacji limfatycznych na przykładzie pacjentów DSK UCK WUM
1Department of Paediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Lidia Zawadzka-Głos, MD, PhD
2Department of Paediatric Radiology, Medical University of Warsaw, Poland
Head of Department: Michał Brzewski, MD, PhD
3II Department of Clinic Radiology, Medical University of Warsaw, Poland
Head of Department: Professor Olgierd Rowiński, MD, PhD
Streszczenie
Wstęp. Malformacje limfatyczne (ML) są zmianami łagodnymi, powstałymi w wyniku nieprawidłowego formowania naczyń limfatycznych w życiu płodowym. Najczęstszą lokalizacją ML są obszary głowy i szyi. Ze względu na swoją morfologię i lokalizację w pobliżu istotnych struktur naczyniowych i nerwowych, leczenie chirurgiczne jest trudne, obarczone wysokim ryzykiem powikłań i często niedoszczętne.
Cel pracy. Skleroterapia z użyciem bleomycyny jest uznaną małoinwazyjną metodą leczenia ML. W pracy przedstawiamy wyniki leczenia ML okolicy głowy i szyi przy użyciu bleomycyny u dzieci z naszego ośrodka.
Materiał i metody. Od września 2017 do października 2019 roku leczeniu z użyciem bleomycyny poddanych zostało 6 pacjentów w wieku od 3 tygodni do 10 lat z ML głowy i szyi. Zabiegi wykonywano pod kontrolą USG i/lub fluoroskopii. Przeanalizowano liczbę procedur u pacjenta, dawki leku oraz odpowiedź na leczenie i powikłania.
Wyniki. U 4 pacjentów ML była zlokalizowana na szyi, u jednego na szyi i w śródpiersiu, u jednego w okolicy policzka. Dwóch pacjentów zostało poddanych jednemu zabiegowi, u 3 pacjentów wykonano 2 zabiegi, u jednego 3 zabiegi. U 4 pacjentów efekt leczenia był doskonały, u jednego dobry. Efekt leczenia u jednego pacjenta po pojedynczej dawce leku był niezadowalający, dlatego został on zakwalifikowany do kolejnego etapu. Maksymalna jednorazowa dawka bleomycyny wyniosła 10 000 j., nie przekraczano 700 j./kg mc. Nie odnotowano powikłań pozabiegowych.
Wnioski. Wstępne wyniki sugerują, że skleroterapia ML okolicy głowy i szyi przy użyciu bleomycyny u dzieci jest efektywną i bezpieczną metodą leczenia.
Summary
Introduction. Lymphatic malformations (LMs) are benign lesions thought to be caused by the abnormal development of the lymphatic system in utero. Most commonly, LMs affect the head and neck. Because of LM morphology and location close to important vascular and nervous structures, surgical treatment is difficult, associated with a high risk of complications, and often incomplete.
Aim. Bleomycin sclerotherapy is a recognised minimally invasive technique used in the treatment of LMs. We present the outcomes of bleomycin therapy of LMs located in the head and neck area in children receiving therapy in our centre.
Material and methods. Between September 2017 and October 2019, treatment with bleomycin was provided to a total of 6 patients with LMs of the head and neck, aged from 3 weeks to 10 years. The procedures were performed under ultrasound and/or fluoroscopy guidance. The aspects analysed included the number of procedures applied in patients, drug doses, treatment response and complications.
Results. In 4 patients, the LM was located on the neck, in 1 patient ? on the neck and in the mediastinum, and in 1 patient in the cheek region. Three patients underwent 2 procedures, 1 patient ? 3 procedures, and 2 patients ? 1 procedure. The treatment outcome was excellent and good in 4 patients and 1 patient, respectively. However, in 1 patient, the therapeutic effect was unsatisfactory, and a decision was made to administer another course of treatment. The maximum single dose of bleomycin was 10,000 IU; the dose of 700 IU/kg BW was not exceeded. No complications were observed after the procedures.
Conclusions. Preliminary results suggest that bleomycin sclerotherapy of LMs in the head and neck region in children is an effective and safe treatment modality.
Introduction
Vascular anomalies in children are common developmental disorders. Their presentation varies from small, spontaneously resolving lesions to extensive life-threatening abnormalities which cause lasting disfigurement and severe functional impairment. Based on the clinical picture and histological structure, the International Socie-ty for the Study of Vascular Anomalies (ISSVA) classifies vascular anomalies into two categories: 1) vascular tumours (mostly infantile haemangiomas) and 2) vascular malformations (1).
Vascular malformations and haemangiomas have different clinical manifestations. Firstly, malformations are present from birth and grow with the individual. They do not regress, and do not have a tendency to become malignant. They occur with similar frequency in both sexes.
A comparison of the characteristics of haemangiomas and malformations is given in table 1 (2).
Tab. 1. A comparison of the characteristics of haemangiomas and malformations
| Vascular malformation | Haemangioma |
| Clinical characteristics |
? Always present at birth
? Grows in proportion to the child’s growth
? Never resolves
? Prevalence ?:? 1:1 | ? Occurs in the neonatal or early infantile period
? Grows faster than the growth of the child during the 1st year
? Involutes after 1 year of age
? Prevalence ?:? 1:6 |
| Biological characteristics |
? Flat, normal endothelial cells; normal mast cell count
? Involution: normal endothelial cell turnover; dysplastic vascular channels: venules, veins, capillaries or lymphatic vessels
? | ? Proliferation: abundant, proliferative endothelial cells, numerous mast cells
? Involution: apoptosis and progressive degrees of flattening of endothelial cells Wider but less numerous vessels surrounded by perivascular fibrous fatty tissue |
Vascular malformations are benign tumour-like lesions that occur at 4-10 weeks of gestation as a result of abnormalities in the development of vascular tissue. They are characterised by normal cell turnover, and do not undergo spontaneous involution (2, 3).
Depending on the type of vascular channel, they are divided into several categories including: simple vascular malformations: capillary (CM), lymphatic (LM), venous (VM), arterial (AM), and arteriovenous (AVM); mixed vascular malformations: CVM, CLM, LVM, CLVM, AVM-LM, and CM-AVM; arteriovenous fistulas (AVF) (1).
Depending on the rate of blood flow through the lesion, vascular malformations are divided into two subtypes: low-flow (composed of venous, capillary and lymphatic components), and high-flow (composed of arterial components) (2).
Lymphatic malformations, previously referred to as lymphangioma or cystic hygroma, are low-flow malformations. They are composed of malformed dysplastic vessels filled with lymphatic fluid, presenting as dilated channels, and lymphatic cysts (2, 3). Typically, they cause enlargement of the involved organ. They can be located in any major site of lymphatic tissue. Macrocystic malformations (> 2 cm in diameter) are large, soft, smooth clear masses under normal skin. Microcystic malformations (< 2 cm) penetrate the skin or muscles, and present as thin vesicles in the skin or muscle tissue (2). Mixed malformations have both components (the most common type). Macrocystic LMs respond better to sclerotherapy.
The primary locations for LMs include the head and neck region (50-75%), most commonly the posterior triangle of the neck, but also the bottom of the oral cavity as well as the mediastinum, larynx and tongue (2, 3). The second most common location is the torso (chest, axillary fossa) and the extremities. Visceral locations are rare. Malformations may increase in volume significantly during upper respiratory tract infections due to the accumulation of fluid, but also during inflammation, after an injury or haemorrhage into the lesion, with possible effects including airway obstruction or bleeding, and exacerbation of existing problems, e.g. with swallowing or head mobility ? depending on the site of the lesion. Because of specific morphology and location close to important vascular and nervous structures, surgical treatment is difficult, associated with a high risk of complications, and often non-radical.
Vascular malformations are always present at birth. They can be identified by prenatal ultrasound. Despite their congenital nature they may remain clinically silent until puberty and adulthood. They may appear individually or in clusters, as in Klippel-Trenaunay syndrome (KTS) and CLOVES (Congenital Lipomatous Overgrowth, Vascular malformations, Epidermal nevi and Skeletal/Spinal deformities syndrome) (2, 3).
In rare cases, familial occurrence has been reported (two families with congenital venous malformations and a specific mutation within the VMCM-1 gene located on the chromosome 9p) (2), and lymphatic malformations located in the same site in the child and the mother (4).
Diagnostic work-up
The diagnosis is largely based on patient history, clinical findings and radiological evaluation. The most useful diagnostic modalities are Doppler ultrasound and MRI with contrast.
With Doppler ultrasound, lesions are identifiable already in the first trimester of pregnancy (especially macrocystic malformations) and at birth (3, 5). Doppler ultrasound shows an avascular hypoechogenic cystic structure with visible fluid levels.
MRI with contrast as a complementary examination to ultrasound scanning (3, 5) additionally evaluates the extent of the lesion and its position relative to the surrounding structures. T2 images reveal iso- or hyperintense cystic lesions with a characteristic peripheral enhancement. A lobular structure or septa between cysts can be seen especially in macrocystic lesions (3).
The management of lymphatic malformations is often challenging to the treating physician. Small, limited and superficial lesions can be treated surgically with good results. However, the surgical treatment of extensive lesions poses a greater challenge. The following therapeutic modalities have been reported in the literature:
1. Surgical resection (radical, if possible), while conserving important vascular/neural structures (microcystic lesions). Multi-stage surgical treatment in most cases, treatment (frequently non-radical) especially in the suprahyoid location (recurrence rate 85%; high risk of complications) (2, 3, 6),
2. Laser therapy ? CO2, Nd:YAG in limited mucosal lesions.
3. Observation (postinfectious “autosclerotherapy” process).
4. Aspiration ? ad-hoc management (haemorrhaging, infections).
5. Sclerotherapy ? obliteration of low-flow cystic lesions by intralesional administration of alcohol, doxacillin, cyclophosphamide, acetic acid, triamcinolone, OK-432 (Picibanil) or bleomycin (3, 6-8). Vascular endothelial damage results in decreased fluid production, fluid absorption, formation of adhesion and fibrosis, ultimately leading to a reduction in the volume of the malformation (3, 6-8). It represents a minimally invasive first-line therapy. The method is both more efficient and more effective, and furthermore it is associated with a lower risk of complications than surgical management (6). Partial aspiration of the fluid content prior to the intralesional injection of bleomycin has been shown to yield good results. The best effects are observed in the treatment of macrocystic LMs (3).
Bleomycin is an antibiotic chemotherapeutic agent with anti-cancer activity. One of the side effects of bleomycin is its destructive activity on vascular endothelial cells through non-specific inflammatory response, which causes fibrosis and closure of the malformation (6-9). Bleomycin is commonly used as a substance closing off the spaces within lymphatic malformations.
Aim
The aim of the study was to review the effects of treatment of lymphatic malformations in the head and neck region by intralesional bleomycin injections.
Material and methods
A total of 6 children with diagnosed lymphatic malformations in the head or neck region were hospitalised in the Department of Paediatric Otolaryngology, Medical University of Warsaw, between September 2017 and October 2019, to receive intralesional bleomycin injection treatment. The aspects analysed in the study included the age and sex of the patients, the number of procedures applied, as well as drug dose, treatment response and complications. At the start of treatment, the youngest patient was 3 weeks old, and the oldest 10 years and 10 months old. The group consisted of 4 girls and 2 boys.
The patients were selected for treatment on the basis of lymphatic malformation diagnosed by ultrasound and MRI. The procedures were performed under ultrasound and/or fluoroscopy guidance (fig. 1, 2).

Fig. 1. Lymphatic malformation in fluoroscopy. Photo after contrast administration. Patient S.N.

Fig. 2. Lymphatic malformation in fluoroscopy. Photo after contrast administration. Patient W.I.
In 4 patients, the lesion site was on the neck. In 1 patient, the malformation was located on the neck and in the mediastinum, and in another patient it was present in the cheek region.
The technique involves the injection of bleomycin prepared in the sterile drug laboratory in syringes, diluted with saline to 5 ml of the solution, into the lumen of the lymphatic malformation under ultrasound visualisation as well as/or X-ray scopy. The procedures are performed under general anaesthesia because of their long duration and the lack of patient cooperation.
Follow-up ultrasound scanning is carried out at least 6 weeks after the procedure. If necessary, the next stage of treatment is scheduled after at least 2 months of follow-up.
Results
1. Three patients underwent 2 procedures, 2 patients ? 1 procedure, and 1 patient ? 3 procedures. The treatment outcome was excellent in 3 patients, good in 2 patients and unsatisfactory in 1 patient.
2. The maximum single dose of bleomycin was 10,000 IU; the dose of 700 IU/kg BW was not exceeded.
No immediate perioperative or distant postoperative complications were noted.
Patient G.J.
MRI findings (August 2017): an extensive lesion with multiple chambers is seen laterally on the right side of the neck below the submandibular salivary gland, directly under the platysma muscle; from the medial side, the lesion partially adheres to the to the right lobe of the thyroid gland; laterally and anteriorly, it encircles the right CCA and adheres to its bifurcation into the ICA and ECA, and the internal jugular vein; at the boundary between the oropharynx and the laryngopharynx, it projects with a narrow (approx. 3 mm) section into the retropharyngeal space, where it forms a lesion measuring 36 x 13 x 7 mm extending into the laryngopharynx (fig. 3).
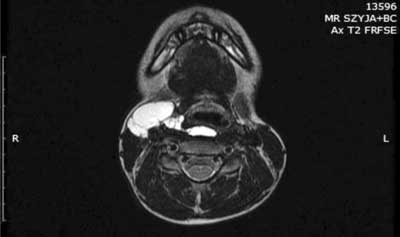
Fig. 3. The picture of MRI examination of M.K. patient. Study description in the text
The patient was considered eligible to undergo treatment to obliterate the lesion. Bleomycin at a dose of 4,500 IU was administered intralesionally. As a result, the superficial part of the malformation was decreased in size.
Follow-up ultrasound scanning performed in September 2018 revealed the presence of a cavity measuring 24 x 12 x 46 mm on the right side of the neck at the level of the bulb. The cavity, filled mainly by thick fluid moving after applying compression, was located in the immediate vicinity of the salivary glands, the right thyroid lobe, and the great vessels, extending into the prevertebral space. After 13 months, the patient was considered eligible for repeat obliteration of the lesion. This time, bleomycin was injected into the deep compartment of the malformation at a dose of 8,000 IU. The final follow-up examination in September 2019 showed residual fluid-filled cavities along the cervical vessels. A decision was made to wait until the next follow-up examination in 3 months.
Patient W.I.
Lymphatic malformation within the area of the neck, the supraclavicular region, and the left side of the nape. The patient was considered eligible to undergo treatment to obliterate the lesion. Bleomycin at a dose of 3,500 IU was administered to the major compartments of the lesion. The final follow-up ultrasound performed 6 months after the procedure showed a residual fragment of the malformation. Another follow-up was scheduled in 6 months (fig. 4, 5).

Fig. 4. The patient W.I. Photo before lymph malformation therapy with bleomycin

Fig. 5. The patient W.I. Photo after lymph malformation therapy with bleomycin
Patient M.K.
MRI findings (March 2019) (fig. 6): multiple small fluid-filled spaces with fluid collection in the left parotid gland from the level of angle of the mandible to the base of the skull; fluid-filled spaces located mainly in the deep lobe of the parotid gland; the left parapharyngeal space is deformed and slightly displaced anteriorly and medially. The patient was considered eligible for bleomycin treatment, and the drug was administered via two injections in the inferior part of the malformation (total dose: 1,000 IU). During the final follow-up examination performed 6 months after the procedure, ultrasound scanning revealed small fluid-filled spaces in the region of the left parotid gland.
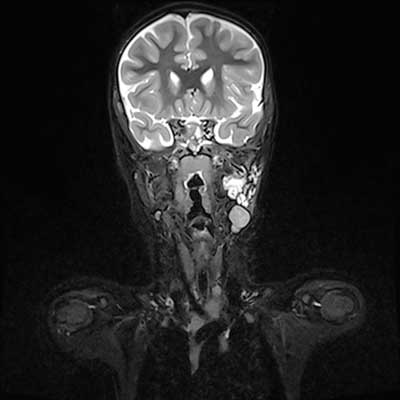
Fig. 6. The picture of MRI examination of M.K. patient. Study description in the text
Patient K.J.
MRI findings (October 2018): a well-circumscribed fluid collection visible within the left masseter muscle, filled with fluid of heterogeneous intensity, measuring 25 x 42 x 50 mm; phleboliths/posthaemorrhagic foci seen in the inferior pole of the lesion, 8-12 mm in diameter; contrast administration enhances the area located peripherally in the superior segment, measuring 20 x 15 mm, and isolated incomplete septa; the findings are consistent with lymphatic venous malformation. During the procedure, the needle was placed into the deep part of the malformation and, as it was gradually withdrawn, bleomycin was also administered to the superficial layer of the lesion at a total dose of 8,000 IU. Because of unsatisfactory results after the first stage of treatment, the patient required repeated administration of bleomycin after 5 months. Consequently, in the second stage of treatment, bleomycin at a dose of 10,000 IU was administered intralesionally. However, a follow-up ultrasound performed 4 months later revealed no significant improvement. The next follow-up ultrasound was scheduled in 3 months.
Patient S.N.
Ultrasound findings (October 2018): a large cystic/solid lesion with multiple fluid cavities of various sizes (including microcystic lesions) is visible on the left side of the neck. The findings are consistent with a macro/microcystic lymphatic malformation encircling the great cervical vessels from the left (fig. 7). The patient was considered eligible for intralesional bleomycin injection treatment, and accordingly bleomycin at a dose of 4,000 IU was administered subcutaneously (fig. 8). Follow-up ultrasounds revealed small micro-type fluid collections in the posterior part of the malformation. The girl was referred for the next stage of treatment, and after 11 months chemoembolisation of the lesion with bleomycin at 10,000 IU was performed. The next follow-up imaging examination was scheduled to be performed 3 months after the last procedure (fig. 9).
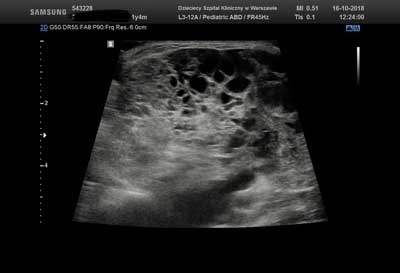
Fig. 7. The picture of ultrasound examination of S.N. patient. Study description in the text
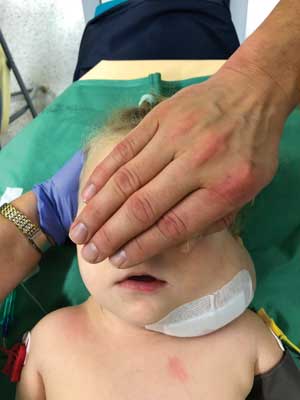
Fig. 8. The patient S.N. Photo before lymph malformation therapy with bleomycin
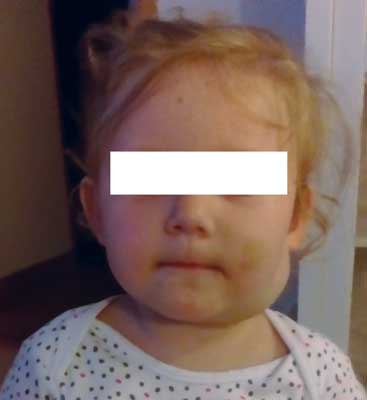
Fig. 9. The patient S.N. Photo after lymph malformation therapy with bleomycin
Patient during the initial procedure and after the second stage of treatment.
Patient O.W.
MRI findings (March 2019): a lesion with a fluid signal, measuring 44 x 45 x 48 mm, is seen in the right supraclavicular region and on the right side of the neck, posteriorly to the sternocleidomastoid muscle; the lesion is thin-walled, with narrow septa inside, enhancing after contrast administration, not penetrating deep into the chest. The patient was referred for chemoembolisation in the third week of life. During the procedure, bleomycin at a dose of 3,000 IU was administered subcutaneously into the lesion. Since a follow-up ultrasound scan showed a residual micro-type malformation, the patient was referred for the next stage of treatment with intralesional injection of bleomycin at a dose of 5,000 IU (fig. 8). The next follow-up ultrasound was scheduled in less than 2 months.
Discussion
Bleomycin is an antibiotic chemotherapeutic agent with anti-cancer activity. One of the side effects of bleomycin is its destructive activity on vascular endothelial cells through non-specific inflammatory response, which causes fibrosis and closure of the malformation (6-9). Bleomycin treatment of lymphatic malformations represents the first-line therapeutic strategy (10). The modality has been shown to be less invasive and more effective than surgical treatment (6, 10). There are literature reports of very good therapeutic outcomes in the treatment of LMs located in the nasopharynx, throat and larynx by applying transmucosal sclerotherapy in fiberoscopy or in direct laryngoscopy under scopic guidance (11, 12). It has been highlighted that the procedure is associated with a low risk of oedema, which is a particularly important aspect in patients with lesions in the upper respiratory tract. Bleomycin sclerotherapy may be a treatment stage preceding surgical resection. The boundaries of the lesion are more easily identifiable, and the procedure itself is associated with a lower risk of bleeding and complications (13).
Short-term side effects occurring around the time of the procedure include nausea, rarely vomiting, allergic reaction and pigmentation of the skin over the lesion. Overall, complications affect 24% of the patients (14).
The most prevalent serious adverse effects associated with bleomycin used as a component of a polychemotherapy protocol in the treatment of cancer and malignant lymphomas is interstitial pneumonia (less commonly localised pneumonia or hypersensitivity pneumonitis) (15) which may occur within 6 months after administration. Interstitial pneumonia develops in about 10% of patients receiving bleomycin, and may lead to pulmonary fibrosis and death in 1% of patients (9, 16).
The risk of pneumotoxicity increases with cumulative doses. It rises considerably at a cumulative dose of 400 IU (9), but it may also occur at lower doses.
Risk factors for lung fibrosis include elderly age, liver or kidney dysfunction, history of pulmonary disorders, previous lung irradiation, smoking and oxygen therapy. 100 percent oxygen therapy must not be used in functional pulmonary tests and during anaesthesia (9, 16). Instead, 21 percent oxygen should be applied.
Importantly, there are no reports of pulmonary fibrosis associated with bleomycin administration directly into the lymphatic malformation. In the literature, there is a single reported case of acute toxic pneumonitis after a low dose of bleomycin administered directly into the venous malformation (17). It was the second application of the drug in that patient, and she was successfully treated with steroid therapy.
Also, there has been one report of lung injury after the treatment of venous malformation with a low dose of bleomycin (15 IU/0.75 IU/kg) with lipiodol (18).
Pulmonary function tests, particularly measurements of diffusing capacity for carbon monoxide (DLCO ? a 25% decrease from the baseline) and vital capacity often provide a basis for the early diagnosis of pneumotoxicity (9, 19).
Bleomycin treatment should be discontinued immediately, if the patient has unexplained cough, dyspnoea, crackling at the lung base, or disseminated lesions with a reticular pattern seen on chest X-ray (9).
Conclusions
Preliminary results suggest that bleomycin sclerotherapy of LMs in the head and neck region in children is an effective and safe treatment modality. There are no literature reports of pulmonary fibrosis occurring during LM treatment by bleomycin sclerotherapy. The reported efficacy of LM treatment with a single bleomycin sclerotherapy procedure seems worthy of note (20).
Piśmiennictwo
1. ISSVA classification for vascular anomalies. Approved at the 20th ISSVA Workshop, Melbourne, April 2014, last revision May 2018.
2. Wyrzykowski D, Bukowski M, Jaśkiewicz J: Guzy naczyniowe i wrodzone malformacje naczyniowe. Cancer Surgery 2009; 1: 1-17.
3. Nassiri N, Thomas J, Cirillo-Penn NC: Evaluation and management of peripheral venous and lymphatic malformations. J Vasc Surg Venous Lymphat Disord 2016; 4(2): 257-265.
4. Gupta A, Sardana K, Arora P et al.: Microcystic lymphatic malformation in a child and his mother. Pediatr Dermatol 2019; 36(6): 967-969.
5. Lerat J, Bisdorf-Bresson A, Borsic M et al.: Guidelines of the French Society of Otorhinolaryngology on Cervical Lymphatic malformation in adults and children: Diagnosis. Eur Ann Otorhinolaryngol Head Neck Dis 2019; 136(2): 109-112.
6. Ardıçlı B, Karnak ?, Çiftçi AÖ et al.: Sclerotherapy with bleomycin versus surgical excision for extracervical cystic lymphatic malformations in children. Surg Today 2016; 46(1): 97-101.
7. Bhatnagar A, Upadhyaya VD, Kumar B et al.: Aqueous intralesional bleomycin sclerotherapy in lymphatic malformation: Our experience with children and adult. Natl J Maxillofac Surg 2017; 8(2): 130-135.
8. Sindel A, Sayan A, Özgür Ö et al.: Percutaneous treatment of orofacial vascular malformations. Br J Oral Maxillofac Surg 2018; 56(3): 206-211.
9. Blemycin CHPL.
10. Vlahovic A, Gazikalovic A, Adjic O et al.: Bleomycin sclerotherapy for lymphatic malformation after unsuccessful surgical excision: case report. Acta Otorhinolaryngol Ital 2015; 35(5): 365-367.
11. Oomen KP, Paramasivam S, Waner M, Niimi Y: Endoscopic transmucosal direct puncture sclerotherapy for management of airway vascular malformations. Laryngoscope 2016; 126(1): 205-211.
12. Chen J, Li W, Li X: Retropharyngeal lymphatic malformations: report of two successfully treated cases and review of the literature. Acta Otorhinolaryngol Ital 2019; 39(3): 205-209.
13. MacArthur CJ, Nesbit G: Simultaneous intra-operative sclerotherapy and surgical resection of cervicofacial venous malformations. Int J Pediatr Otorhinolaryngol 2019; 118: 143-146.
14. Mack JM, Richter GT, Becton D et al.: Short-term side effects and patient-reported outcomes of bleomycin sclerotherapy in vascular malformations. Pediatr Blood Cancer 2018; 65(6): e27008.
15. Camus P: Interstitial lung disease from drugs, biologics, and radiation. [In:] Schwarz MI, King TE Jr (eds.): Interstitial Lung Disease. 5th ed. People’s Medical Publishing House, Shelton, CT 2011: 637.
16. Jules-Elysee K, White DA: Bleomycin-induced pulmonary toxicity. Clin Chest Med 1990; 11: 1.
17. Mèndez-Echevarría A, Fernandez-Prieto A, de la Serna O: Acute Lung Toxicity After Intralesional Bleomycin Sclerotherapy. Pediatrics 2018; 141(1). pii: e20161787.
18. Khera D, Bhatnagar A, Khera PS, Kumar P: Percutaneous Sclerotherapy of Superficial Vascular Malformation Leading to Bleomycin Induced Lung Injury and Lipoid Pneumonia. Indian J Pediatr 2019; 86(1): 97-98.
19. Lauritsen J, Kier MG, Bandak M et al.: Pulmonary Function in Patients With Germ Cell Cancer Treated With Bleomycin, Etoposide, and Cisplatin. J Clin Oncol 2016; 34: 1492.
20. Upadhyaya VD, Bhatnagar A, Kumar B et al.: Is multiple session of intralesional bleomycin mandatory for complete resolution of macrocystic lymphatic malformation? Indian J Plast Surg 2018; 51(1): 60-65.








