© Borgis - New Medicine 4/2006, s. 122-123
*Zbigniew Juraszyński1, Jerzy Pręgowski2, Zofia T. Bilińska2, Marcin Demkow2, Dorota Piotrowska-Kownacka3, Witold Rużyłło2
False diagnosis of false left ventricular aneurysm in patient with post-infarction VSD
1First Department of Cardiac Surgery, Institute of Cardiology, Warsaw
Head of department: Prof. Witold Rużyłło
2First Department of Coronary Artery Disease, Institute of Cardiology, Warsaw
Head of department: Prof. Andrzej Biederman
3Nuclear Medicine Department, Warsaw Medical Academy, Warsaw
Head of department: Prof. Grzegorz Opolski
Summary
Summary
A case of 57-year-old man with acute inferior myocardial infarction treated with primary angioplasty of the right coronary artery is reported. The patient was referred for urgent cardiac surgery. After finishing the procedure the patient was discharged from hospital in a good clinical condition.
Rupture of the free wall of the left ventricle is a serious complication of acute myocardial infarction, in most cases leading to death. However, this complication, if not lethal, may sometimes lead to the formation of a left ventricle pseudoaneurysm. There have been several case reports describing patients with this clinical condition [1-3]. The optimal treatment of these patients remains unknown1. The current manuscript will describe the case of a patient with misdiagnosed left ventricle pseudoaneurysm.
A 57-year-old man with acute inferior myocardial infarction was treated with primary angioplasty of the right coronary artery. However, no adequate perfusion was restored due to the no-reflow phenomenon, which occurs in up to 30% of primary PTCA and may contribute to rupture of the left ventricle free wall [4-8]. Five days after the procedure the patient developed a new heart murmur. On echocardiography ventricular septal defect (VSD) and pericardial fluid were found. The thickness of the fluid layer was 25 mm with fibrin deposits. Left ventricle postero-inferior wall and interventricle septum were thinned, dyskinetic with aneurysm formation. Overall, echocardiography suggested left ventricle pseudoaneurysm. Cardiac Magnetic Resonance Imaging (MRI) was performed; VSD was confirmed and pseudoaneurysm formation of the inferior wall of the left ventricle was suspected. Pericardial fluid thickness assessed with MRI was 16 mm with clearly visible fibrin deposits. After contrast medium injection, signal enhancement from the pericardial fluid was detected, further supporting evidence for communication between the left ventricle and the pericardial space. The patient was referred for urgent cardiac surgery.
Surgery
The anaesthetic technique included insertion of a radial artery line, central venous line, peripheral venous cannulae, Swan-Ganz catheter and urinary catheter, and administration of fentanyl, dormicum, pancuronium and etomidat, with controlled mechanical ventilation. After performing a median sternotomy, the pericardium was opened longitudinally in an inverted "T” fashion. The posterior aspect of the heart was then examined. After exposure, the diagnosis of left ventricle pseudoaneurysm was not confirmed. True aneurysm of the inferior wall of the left ventricle (4/6 cm) was found.
There were no signs of a tear in the left ventricle. Systemic heparin was administered (3 mg/kg). Cannulation of the aorta and double venous cannulation from the right atrium were performed. A membrane oxygenator and roller pump were used. After aorta crossclamping and administration of blood cardioplegia the left ventricle was opened through the aneurysm and an intraseptal cavity (2/3/4 cm) was found with 3 defects – one large on the left ventricle side (1/3 cm) and 2 small on the right ventricle side, approximately 2 mm each. Both defects of the right ventricle were secured with two polypropylene 4-0 sutures reinforced with Teflon pledgets. The large defect was closed with a Dacron patch. The ventriculotomy was closed with a 3-0 polypropylene running suture reinforced with Teflon pledgets. The patient was weaned from the cardiopulmonary bypass without incident and without the need for inotropic support. After finishing the procedure the patient was transferred to the intensive care unit (ICU) in a haemodynamically stable condition.
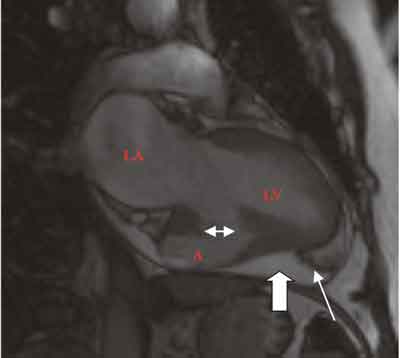
Fig. 1. Long axis MRI cross-section of left ventricle and left atrium. Aneurysm/pseudoaneurysm involves half of the inferior wall. Pericardial fluid layer is indicated with thick arrow, fibrin deposits with thin arrow. Double head arrow points at remnants of LV wall. LA – left atrium, LV – left ventricle, A – aneurysm/pseudoaneurysm.
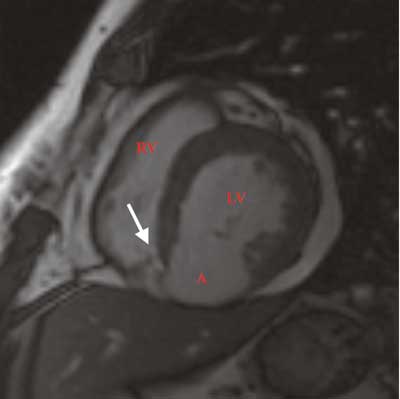
Fig. 2. Short axis MRI cross-section of left ventricle and right ventricle. Pericardial fluid is visible. LV – left ventricle, RV – right ventricle, A – aneurysm/pseudoaneurysm. The arrow indicates VSD.
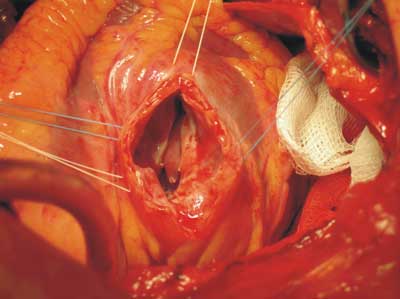
Fig. 3. Intra-operative picture of ventricular septal defect. The free wall of left ventricle at aneurysm site is cut.
Postoperative course was uneventful. The patient was extubated within 12 hours. Blood product transfusion was unnecessary, because the total amount of blood loss was about 450 ml. The next day the patient was moved to the ward in a stable condition. He was discharged from hospital within 14 days in a good clinical condition. Seven months after the surgery the patient remains in a good condition.
Piśmiennictwo
1. Moreno R, Gordillo E, Zamorano J, et al. Long term outcome of patients with postinfarction left ventricular pseudoaneurysm Heart 2003;89:1144-1146. 2.Burger A, Sherman H. Left Ventricular Pseudoaneurysm. N Engl J Med 2001; 344(25): 1910. 3.Sřrensen M, Moat N, Mohiaddin R et al. False Left Ventricular Aneurysm Documented by Magnetic Resonance Imaging. Circulation. 2002;105:1734. 4.Morishima I, Sone T, Mokuna S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130(2):239-43. 5.Iwakura K, Ito H, Ikushima M, et al. Association Between Hyperglycemia and the No-Reflow Phenomenon in Patients With Acute Myocardial Infarction J Am Coll Cardiol 2001;38:472-7. 6.Ito H, Maruyama A, Iwakura K, et al. Clinical implications of ´no-reflow´ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 1996;93:223-8. 7.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty Am Heart J 1995;130:239-43. 8. Mehta R, Harjai K, Boura J, et al. Prognostic Significance of Transient No-Reflow During Primary Percutaneous Coronary Intervention for STElevation Acute Myocardial Infarction Am J Cardiol 2003;92:1445-1447.


