Agnieszka Kozubska1, Danuta Chlebna-Sokół2, Elżbieta Jakubowska-Pietkiewicz3, Izabela Michałus3, Karolina Beska-Bartecka4, *Joanna Szczepańska5
The oral health status and dental treatment needs of children affected by osteogenesis imperfecta – part I
Stan jamy ustnej oraz stomatologiczne potrzeby lecznicze dzieci dotkniętych osteogenesis imperfecta
1Doctoral studies, Department of Paediatric Dentistry, Medical University of Łódź, Poland
Head of Department: Professor Joanna Szczepańska, MD, PhD
2University Pediatrics Center M. Konopnicka, Central Clinical Hospital of Medical University of Łódź, Poland
Head of Center: Dr n. med. Monika Domarecka
3Department of Paediatrics, Newborn Pathology and Metabolic Diseases of the Bones, Medical University of Łódź, Poland
Head of Department: Elżbieta Jakubowska-Pietkiewicz, MD, PhD
4Osteoporosis Treatment Center and other Metabolic Diseases of Bones in Children and Adolescent
Head of Center: Danuta Chlebna-Sokół, MD, PhD
5Department of Paediatric Dentistry, Medical University of Łódź, Poland
Head of Department: Professor Joanna Szczepańska, MD, PhD
Streszczenie
Wstęp. Osteogenesis imperfecta jest to zespół uwarunkowanych genetycznie chorób tkanki łącznej powstałych na skutek mutacji najczęściej w genach kodujących kolagen typu I – COL1A1. Oprócz objawów ogólnoustrojowych występują także charakterystyczne zaburzenia w układzie stomatognatycznym.
Cel. Celem badania była ocena stanu uzębienia oraz stomatologicznych potrzeb leczniczych dzieci z wrodzoną łamliwością kości.
Materiały i metody. Przeprowadzenie ankiety obejmującej wywiad ogólnolekarski oraz stomatologiczny z rodzicami dzieci z OI. Wykonanie badania stomatologicznego uwzględniającego stan uzębienia, występowanie dentinogenesis imperfecta, ocena stopnia starcia wg współczynnika Broca, nieprawidłowości zgryzu, wartości wskaźnika puw/PUW.
Wyniki. Przebadano łącznie 62 osoby z wrodzoną łamliwością kości, w tym 32 chłopców i 30 dziewczynek. Najczęściej opisano prawidłowy okres wyrzynania zębów mlecznych – u 48 osób (77,42%) i zębów stałych – u 34 osób (82,93%). U większości pacjentów zaobserwowano nieprawidłowe nawyki higieniczne i żywieniowe. Dentinogenesis imperfecta zębów mlecznych i stałych najczęściej występowało w typie III OI. Uzyskane wartości wskaźnika puw/PUW dla badanych pacjentów z OI kształtowały się na znacznie niższym poziomie niż aktualne dane epidemiologiczne.
Wnioski. Pomimo złych nawyków żywieniowych i higienicznych oraz patologicznej budowy uzębienia pacjentów z OI, wskaźniki próchnicy u tych dzieci, w porównaniu z badaniami populacyjnymi odpowiednich grup wiekowych, kształtowały się na niskim poziomie.
Summary
Introduction. Osteogenesis imperfecta is an inherited disorder of the connective tissue. Which in most cases, is caused by mutations in the genes encoding collagen type I. Apart from clinical features, there are characteristic dental aberrations.
Aim. The purpose of this research was the assessment of the condition of teeth and therapeutic needs of children with the congenital brittle bone disease.
Material and methods. The questionnaires with patient’s parents, consisting medical history and dental history were performed. The intra-oral examination included the condition of the dentition, the presence of dentinogenesis imperfecta, malocclusion, the assessment of the attrition index and dmft/DMFT index.
Results. 62 patients with osteogenesis imperfecta were examined – 32 boys and 30 girls. There were normal eruption times of deciduous (48 patients – 77.42%) and permanent teeth (34 patients – 82.93%) reported in the majority of the patients with OI. In most cases bad eating and hygienic habits were observed. Dentinogenesis imperfecta in deciduous and permanent teeth was reported mostly in type III of OI. dmft/DMFT index among children with OI were low in comparison to the population studies of corresponding age groups.
Conclusions. Despite bad eating and hygienic habits as well as pathological structure of dentition of patients with the congenital brittle bone disease, caries index among these children were low in comparison to the population studies of corresponding age groups.
Introduction
Osteogenesis imperfecta (OI) is a rare inherited disorder caused by mutations most often in genes encoding collagen type I – COL1A1 and COL1A2, or less frequently in genes encoding the proteins involved in the biosynthesis of collagen type I. It is inherited in an autosomal dominant manner, less often in autosomal recessive or associated with the chromosome X (1-3). There is no predilection for gender and race. It occurs at a frequency of 6-7/100,000 births. On “The list of the rare diseases known in Poland” it takes 290th place out of 410 (4-6).
The David Sillence’s (7) classification from 1979, distinguishes four types of OI. It was created on the basis of clinical and radiological symptoms (i.e. the severity of fractures and the deformations of the skeletal system, the color of sclerae, hearing disorders) and the method of inheritance (8). Children with this disorder are characterized by reduced bone mass and density, which makes them fragile. As a consequence, it leads to repeated fractures, inadequate to the strength of the injury, and deformation of the long bones. Patients with osteogenesis imperfecta may also experience growth disorders, laxity of joints and ligaments, and dental aberrations (1, 9).
The characteristic features of the stomatognathic system include dentinogenesis imperfecta type I (DGI), the presence of malocclusion, hipodontia, and ectopic eruption of permanent teeth. DGI affects both the deciduous and permanent dentition, however, more severe changes in the primary dentition. There are tooth color disorders (from brown to opalescent blue/gray). There is often the enamel loss as a result of abnormal dentin structure, exposing the dentin and its rapid abrasion to the gingival level (10-12). A significant abrasion can happen in a very short time. The studies of Levin et al. (13) and Schwartz and Tsipouras (14) show that enamel loss is not caused by an abnormal structure of the enamel-dentin junction, but by an abnormal structure of the dentin, therefore a fracture occurs at the junction of the mantle and peribasal dentin. The dentin, visualized through the enamel loss, is often shiny and hard, as a result of dentinal tubules’ obliteration. Radiological examination shows barrel-shaped crowns, thin and short roots, and obliterated pulp canals (12, 15).
Aim
The purpose of this research was the assessment of the condition of the teeth and therapeutic needs of children with the osteogenesis imperfecta.
Material and methods
In 2015-2018, 62 out of 163 patients with osteogenesis imperfecta of the Department of Paediatrics, Newborn Pathology and Metabolic Diseases of the Bones of the Central Clinical Hospital – University Center of Paediatrics in Łódź were examined. Children in age: 6 months to 18 years old were qualified for the study. The study was performed in all children being in the hospital, whose parents consented to an interview and clinical examination. Patients were divided into four groups on the base of the type of osteogenesis imperfecta, according to the Sillence classification (tab. 1).
Tab. 1. Distribution of patients by type of OI
| The type of OI | Number of boys | Number of girls | Totality | % |
| I | 14 | 12 | 26 | 42 |
| II | 2 | 0 | 2 | 3 |
| III | 7 | 13 | 20 | 32 |
| IV | 9 | 5 | 14 | 23 |
A questionnaire with the parent/legal guardian of the child, consisting of three parts – family, medical and dental history was conducted. The first part included questions about the patient’s personal data and the pregnancy. The medical history took into account the child’s previous and current diseases, data on the course of OI: type, age at which the disorder was diagnosed and the diagnosing manner (clinical diagnosis/genetic testing), the presence of OI in family members, the current systemic treatment, as well as accompanying general symptoms (number of bone fractures suffered by patients throughout their lives, body height, occurrence of hearing impairment, joint laxity). The correctness of body height was assessed on the basis of percentile grids (prepared on the basis of data representative for the population of children and adolescents in Poland). The data was obtained in the course of the implementation of OLAF and OLA projects coordinated by the Institute “Children’s Memorial-Health Center” in years 2007-2012 (16, 17).
The third part of the questionnaire included questions about the time of eruption of deciduous/permanent teeth and about hygienic and nutritional habits. The onset of primary tooth eruption before 5 months of age was classified as premature, and after 12 months as delayed. In the case of permanent teeth, there is premature eruption before the age of 5, and delayed after the age of 8, respectively. The physical examination was performed in the hospital and it concerned the assessment of systemic parameters characteristic of OI, such as the color of the sclera and the triangular shape of the face.
Then, the condition of the oral cavity was analyzed with the use of diagnostic instruments used in dentistry (mirror, dental probe) in artificial lighting in patients sitting with their head supported. The criteria of dental condition were based on WHO guidelines (18). Particular attention was paid to the presence of dentinogenesis imperfecta in deciduous and permanent dentition, tooth color, enamel structure and the degree of tooth abrasion, which were assessed using the four-stage Broc index (19). Grade 1 indicates the presence of wear shields. The second degree is the exposed dentin islets. The large visible surface of the dentin is described as grade III. Stage IV is the reduction of the tooth crown due to abrasion. The level of dmft/DMFT index was calculated, no radiographs were taken. The occurrence of malocclusion was analyzed.
Results
A total of 62 people with osteogenesis imperfecta were examined, including 32 boys and 30 girls. The most numerous group were patients with type I of osteogenesis imperfecta, followed by type III, IV, and only two patients with lethal type II were examined. The correct period of primary tooth eruption was described in 48 people (77.42%), premature eruption in 9 (14.52%), and delayed in 5 (8.06%). The eruption of permanent teeth on time was found in 34 patients (82.93%), premature in 4 (9.76%), delayed in 3 (7.32%) (tab. 2).
Tab. 2. The beginning of the eruption of deciduous and permanent teeth in different types of OI
| The type of dentition | The beginning of the eruption | Type I | Type II | Type III | Type IV | Total n/% |
| Primary | before 5. month | 4 | 0 | 4 | 1 | 9/14.52 |
| in 6.-11. month | 22 | 1 | 12 | 13 | 48/77.42 |
| after 12. month | 0 | 1 | 4 | 0 | 5/8.06 |
| Permanent | before 5. year | 1 | – | 3 | 0 | 4/9.76 |
| in 6.-7. year | 17 | – | 8 | 9 | 34/82.93 |
| after 8. year | 0 | – | 1 | 2 | 3/7.32 |
The following data were obtained from the questionnaire questions concerning hygiene and eating habits – 13 patients with primary and mixed dentition fell asleep while bottle-feeding (23.2%), 52 people eat sweets between meals (83.87%), 46 people drink sweetened drinks during the day (74.19%). Parents of 19 surveyed patients stated in the questionnaire that they started brushing their children teeth at 6 months of age (30.65%), parents of 25 children in the first year of life (40.32%), 6 children in the second year of life (9,68%), 12 children in the third year of life (19.35%), 15 patients brush their teeth once a day (24.19%), 46 twice a day (74.19%), 1 patient more than 2 times a day (1.62%) (tab. 3).
Tab. 3. Dietary and hygienic habits of patients with osteogenesis imperfecta
| Eating habits |
| | Yes | No |
| n | % | n | % |
| Eating sweets between meals | 52 | 83.87 | 10 | 16.13 |
| Drinking sweetened drinks | 46 | 74.19 | 16 | 25.81 |
| Falling asleep while bottle-feeding | 13 | 23.2 | 43 | 76.8 |
| Hygienic habits |
| The beginning of teeth brushing | in 6. month of life | in 1. year of life | in 2. year of life | in 3. year of life |
| n | % | n | % | n | % | n | % |
| 19 | 30.65 | 25 | 40.32 | 6 | 9.68 | 12 | 19.35 |
| The frequency of teeth brusing | once a day | twice a day | more then twice a day |
| n | % | n | % | n | % |
| 15 | 24.19 | 46 | 74.19 | 1 | 1.62 |
Dental examination of children with osteogenesis imperfecta showed the presence of dentinogenesis imperfecta in primary dentition in 12 patients (66.67%) with type III, 5 (41.67%) with type IV, 5 (26.32%) with type I and less frequent in permanent dentition in 8 patients (66.67%) with type III and 1 person (5.56%) with type I (tab. 4). The dentition affected by DGI was more often amber colored – in 17 people with primary dentition and 5 people with permanent dentition (fig. 1). Higher degrees of tooth abrasion according to Broc were observed in primary dentition (fig. 2). The most common findings were IV° (in 7 people), successively I° (in 6), III° (in 5) and II° (in 4) (tab. 5). The permanent teeth affected by DGI showed I° of abrasion. The obtained dmft/DMFT values for the studied patients with OI were much lower than the current epidemiological data (tab. 6) (19). There was no evidence of active caries in patients with dentinogenesis imperfecta. In this study, occlusal abnormalities were described in 26 patients (42%) and they were most often frontal defects (tab. 7).
Tab. 4. Evaluation of the presence of DGI in patients with osteogenesis imperfecta
| The type OI | DGI | The color |
| Primary teeth | Permanent teeth | Amber | Grey |
| | | Primary teeth | Permanent teeth | Primary teeth | Permanent teeth |
| I | 5 | 1 | 3 | 1 | 2 | 0 |
| II | 0 | 0 | 0 | 0 | 0 | 0 |
| III | 12 | 8 | 9 | 4 | 3 | 4 |
| IV | 5 | 0 | 5 | 0 | 0 | 0 |
| Total | 22 | 9 | 17 | 5 | 5 | 4 |
Tab. 5. Number of children with DGI with the specific degree of tooth abrasion
| The type of OI | The dentition | The degree of abrasion according to Broc’s index |
| I | II | III | IV |
| I | primary | 0 | 2 | 1 | 2 |
| permanent | 1 | 0 | 0 | 0 |
| II | primary | 0 | 0 | 0 | 0 |
| permanent | 0 | 0 | 0 | 0 |
| III | primary | 4 | 2 | 2 | 4 |
| permanent | 8 | 0 | 0 | 0 |
| IV | primary | 2 | 0 | 2 | 1 |
| permanent | 0 | 0 | 0 | 0 |
| Total | primary | 6 | 4 | 5 | 7 |
| permanent | 9 | 0 | 0 | 0 |
Tab. 6. dmft/DMFT in children with OI compared to the epidemiological data
| The age | Primary dentition |
| | dmft/DMFT of patients with OI | Epidemiological data (19) |
| 3 years | 0.14 | 2.4 (2015) |
| 5 years | 0 | 4.7 (2016) |
| 7 years | 0.2 | 5.61 (2016) |
| | Permanent dentition |
| 12 years | 0 | 3.75 (2016) |
| 15 years | 4.67 | 5.75 (2015) |
Tab. 7. Number of children with OI with a given occlusal abnormality
| Anterior-posterior occlusal aberations |
| Class II malocclusion | 11 |
| Class III malocclusion | 0 |
| Vertical occlusal aberrations |
| Open bite | 5 |
| Deep overbite | 1 |
| Transverse occlusal aberrations |
| Overhang bite | 2 |
| Lateral mandibular shift | 1 |
| Cross bite | 2 |
| Other occlusal aberrations |
| Crowding | 4 |
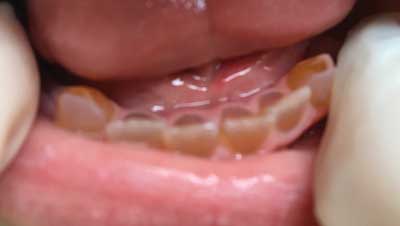
Fig. 1. Patient with OI and DGI of permanent and deciduous teeth. Second degree crowns abrasion. Yellow – brown in color. Own photo
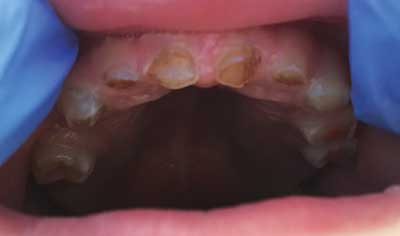
Fig. 2. OI patient with DGI. The abrasion of 3rd and 4th degree crowns. Own photo
Discussion
Sixty-two patients (32 boys and 30 girls) of the Department of Paediatrics, Newborn Pathology and Metabolic Diseases of the Bones of the Central Clinical Hospital – University Center of Paediatrics in Łódź, a leading center in Poland dealing with the treatment of this disorder, were examined. The present study included patients of both sexes aged 6 months to 18 years. The age range was related to the onset of primary tooth eruption in order to be able to assess the condition of the dentition in terms of the presence of DGI. The sexual predilection was not demonstrated, which is consistent with the available literature (4).
According to the available literature (7), type I is the most common (60-70%), type III occurs in 20% of people, and IV in 10%, which is confirmed by the current results – the most numerous group were patients with type I (26 people), then with type III (20 people), IV (14 people) and only two boys with type II were examined. The Sillence classification is currently the most commonly used, although many modifications have been made, in whose the genetic basis was taken into account. In 2009, in Boston, at the conference of International Skeletal Dysplasia Society, Sillence’s criteria were found to be more useful in clinical practice than the genetic criteria (8, 21). During the course of this study, most patients did not have genetic tests indicating the gene in which the mutation occurred (currently most of the respondents have genetic confirmation of osteogenesis imperfecta and these are mutations in the COL1A1 and COL1A2 genes), which was one of the reasons for choosing the Sillence classification. The key reason for this choice, however, was the possibility of comparing the obtained results with those available in the literature.
Dentinogenesis imperfecta type I according to Shields is associated with congenital brittle bone disease. More than 50% of OI patients have DGI (14, 22). However, according to Saeves et al. (23), the frequency of DGI in patients with OI ranges from 19 to 42%. More often dentinogenesis imperfecta is described in types III and IV (23). In our own research, dentinogenesis imperfecta was more often observed in primary dentition, mainly in type III of OI. In the permanent dentition, DGI was more often found in patients with type III and I. Amber color was observed in more patients than opalescent – blue/gray color. Higher degrees of abrasion according to Broc were found in the primary dentition, which indicates a more severe course than in the case of permanent dentition. In the studies by O’Connell and Marini (11), the abrasion wear and enamel fractures were observed mainly in deciduous dentition with a yellow/brown color. On the other hand, studies by Majorana et al. (24) showed no correlation between the type of OI and the color of the teeth affected by DGI.
The performed questionnaire studies showed in most patients incorrect eating habits and hygiene procedures that needed improvement. Despite these results, the dmft/DMFT ratios remained at a low level in relation to the respective age groups as compared to the epidemiological data. The level of caries in children with OI and in children from epidemiological studies carried out under the program “Monitoring of the oral health of the Polish population for 2016-2020” was in the primary dentition – for 3-year-old children, respectively: 0.14 and 2.4; for 5-year-old children: 0 and 4.7; for 7-year-old children: 0.2 and 5.61. Also in adolescents with permanent dentition, the differences between these groups were significant for 12-year-olds: 0 and 3.75, respectively, but they decreased between them at the age of 15 and amounted to 4.67 and 5.75, respectively.
According to Hodge et al. (25), the progression of caries in teeth affected by DGI is slow, mainly due to the rapid wear of abnormally built dentin. According to Shetty et al. (26), teeth affected by DGI are not more susceptible to caries than teeth with normal structure, moreover, the structure of DGI dentin without typical dentinal tubules indicates greater resistance to caries. Similarly, Devaraju et al. (12) believe that caries cannot develop in cases of significant wear due to the absence of dentinal tubules and the absence of enamel.
It should be emphasized that in the examined patients with dentinogenesis imperfecta, no signs of active caries were observed. Perhaps the lack of active lesions in caries or very low values of the dmft/DMFT index in primary dentition, as well as in early permanent dentition, can be attributed to a more advanced form of DGI in these teeth, which, according to literature data, may be a “protective” factor against the demineralisation of origin bacterial.
However, according to Schwartz and Tsipouras (27), in 1/3 of cases, the hypomineralization or hypoplasia of enamel in the course of DGI makes these teeth more susceptible to the development of the caries process. Similarly, Brkić and Pavicin (28) believe that due to the lower content of minerals in teeth with enamel underdevelopment in the presence of DGI, caries spreads faster, attacking the pulp and hence changes in the periapical tissues.
Severe forms of osteogenesis imperfecta are associated with the occurrence of malocclusion. The malocclusion is more often observed in patients with the moderately type of OI, compared to the mild type. According to the literature, skeletal class III is present in 70-80% of people with OI, often coexisting with open bite (11, 28, 29). Similarly, according to Chang et al. (30), a typical feature of forms III and IV of OI is skeletal class III. Schwartz and Tsipouras (27) studied 16 patients with OI, in whom they detected class III in 75%, crossbite in 65%, and impacted teeth in 25%. In this study, occlusal abnormalities were described in 26 patients (42%) and they were mainly class III.
In this study, the eruption periods of the deciduous and permanent teeth were normal in most patients. Despite numerous anomalies in the structure and position of the teeth in patients with osteogenesis imperfecta, no delayed or premature tooth eruption was noted. In contrast, in the studies by O’Connell and Marini (11) delayed eruption was observed in 21% of patients with type III, while accelerated eruption in 23% of patients with type IV. According to Rizkallah et al. (31), delayed or accelerated eruption is a typical dental disorder in the course of osteogenesis imperfecta. According to the studies by Kamoun-Goldrat et al. (32), bisphosphonate therapy causes delayed tooth eruption in OI patients, which was not found in our studies.
Based on the literature and our own observations, the following dental treatment needs of children with osteogenesis imperfecta were developed:
1. Reconstruction of deciduous molars with steel crowns in order to maintain the height of the occlusion.
2. Aesthetic reconstruction of anterior permanent teeth with composite material and, possibly, in the future, the use of prosthetic reconstruction.
3. Change of eating and hygiene habits.
4. Preventive treatments.
5. Treatment of cavities, if necessary.
6. Orthodontic treatment of malocclusion.
Conclusions
Despite poor dietary and hygienic habits and pathological structure of the dentition (abnormal structure of dentin, enamel loss, which leads to rapid abrasion of the crown) in patients with osteogenesis imperfecta, the rates of caries in these children were low compared to the population studies of relevant age groups.
Piśmiennictwo
1. Hoyer-Kuhn H, Netzer Ch, Semler O: Osteogenesis imperfecta: pathophysiology and treatment. Wien Med Wochenschr 2015; 165: 278-284.
2. Morello R: Osteogenesis imperfecta and therapeutics. Matrix Biol 2018; 71-72: 294-312.
3. Borg SA, Bishop NJ: New diagnostic modalities and emerging treatments for neonatal bone disease. Early Hum Dev 2018; 126: 32-37.
4. Huber MA: Osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 103: 314-320.
5. Beska K, Rusińska A, Michałus I, Chlebna-Sokół D: Uwarunkowania genetyczne wrodzonej łamliwości kości – przegląd aktualnego piśmiennictwa. Endokrynol Ped 2014; 3: 57-64.
6. Jakubowska-Pietkiewicz E, Siemiątkowska-Stengert W, Chlebna-Sokół D: Wrodzona łamliwość kości typu II – korzystne zmiany w diagnostyce i leczeniu. Opis przypadku. Post N Med 2016; 29: 734-737.
7. Sillence DO, Senn A, Danks DM: Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 1979; 16: 101-116.
8. Fratzi-Zelman N, Misof BM, Roschger P, Klaushofer K: Classification of osteogenesis imperfecta. Wien Med Wochenschr 2015; 165: 264-270.
9. Pillion JP, Vernick D, Shapiro J: Hearing loss in osteogenesis imperfecta: characteristics and treatment considerations. Genet Res Int 2011; 2011: 1-6.
10. Malmgren B, Norgren S: Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol Scand 2002; 60: 65-71.
11. O’Connell AC, Marini JC: Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol 1999; 87: 189-196.
12. Devaraju D, Devi BY, Vasudevan V, Manjunath V: Dentinogenesis imperfecta type I: a case report with literature review on nomenclature system. J Oral Maxillofac Pathol 2014; 18(suppl. 1): 131-134.
13. Levin LS, Brady JM, Melnick M: Scanning electron microscopy of teeth in dominant osteogenesis imperfecta: support for genetic heterogeneity. Am J Med Genet 1980; 5: 189-199.
14. Schwartz S, Tsipouras P: Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol 1984; 57: 161-167.
15. Barron MJ, McDonnell ST, MacKie I, Dixon MJ: Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis 2008; 3: 31.
16. http://www.czd.pl/index.php?option=com_content&view=article&id=1717&Itemid=538.
17. https://www.mp.pl/pacjent/pediatria/prawidlowyrozwoj/rozwojfizyczny/52272,siatki-centylowe-masa-i-dlugosc-ciala-malego-dziecka.
18. WHO: Oral Health Surveys, Basic Methods of Edition 1986.
19. Panek H: Nasilenie bruksizmu wg własnego wskaz?nika u pacjentów z pełnym uzębieniem naturalnym. Protet Stomatol 2002; 52: 3-8.
20. https://www.gov.pl/web/zdrowie/monitorowanie-stanu-zdrowia-jamy-ustnej-populacji-polskiej-w-latach-2016-2020.
21. Rusińska A, Jakubowska-Pietkiewicz E, Michałus I et al.: Zróżnicowanie objawów klinicznych wrodzonej łamliwości kości – trudności diagnostyczne na podstawie doświadczeń własnych. Post N Med 2016; 29: 716-722.
22. Kaiser-Kupfer MI, Podgor MJ, McCain L et al.: Correlation of ocular rigidity and blue sclerae in osteogenesis imperfecta. Trans Ophthalmol Soc U K 1985; 104: 191-195.
23. Saeves R, Lande Wekre L, Ambjornsen E et al.: Oral findings in adults with osteogenesis imperfecta. Spec Care Dentist 2009; 29: 102-108.
24. Majorana A, Bardellini E, Brunelli PC et al.: Dentinogenesis imperfecta in children with osteogenesis imperfecta: a clinical and ultrastructural study. Int J Paediatr Dent 2010; 20: 112-118.
25. Hodge H, Finn S, Robinson BG: Hereditary opalescent dentin III: histological chemical and physical studies. J Dent Res 1940; 19: 521-536.
26. Shetty N, Joseph M, Basnet P, Dixit S: An integrated treatment approach: a case report for dentinogenesis imperfecta type II. Kathmandu University Medical Journal 2007; 5: 230-233.
27. Schwartz S, Tsipouras P: Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol 1984; 57: 161-167.
28. Brkić H, Pavicin IS: Dental management in osteogenesis imperfecta. Paediatr Croat 2017; 61: 137-140.
29. Bendixen KH, Gjorup H, Baad-Hansen L et al.: Temporomandibular disorders and psychosocial status in osteogenesis imperfecta – a cross-sectional study. BMC Oral Health 2018; 18: 35.
30. Chang PC, Lin SY, Hsu KH: The craniofacial characteristics of osteogenesis imperfecta patients. Eur J Orthod 2007; 29: 232-237.
31. Rizkallah J, Schwartz S, Rauch F et al.: Evaluation of the severity of malocclusion in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofacial Orthop 2013; 143: 336-341.
32. Kamoun-Goldrat A, Ginisty D, Le Merrer M: Effects of bisphosphonates on tooth eruption in children with osteogenesis imperfecta. Eur J Oral Sci 2008; 116: 195-198.

