Anna Turska-Szybka1, Paula Piekoszewska-Ziętek1, Dariusz Gozdowski2, *Dorota Olczak-Kowalczyk1
A survey of the oral lesions in newborns and infants: A two-year cross-sectional study
Zmiany błony śluzowej jamy ustnej u niemowląt: dwuletnie badanie przekrojowe
1Department of Paediatric Dentistry, Medical University of Warsaw, Poland
Head of Department: Professor Dorota Olczak-Kowalczyk, PhD, DMD
2Department of Experimental Statistics and Bioinformatics, Warsaw University of Life Science, Poland
Head of Department: Professor Wiesław Mądry
Streszczenie
Wstęp. Zmiany błony śluzowej jamy ustnej u noworodków i niemowląt mogą być manifestacją różnych chorób, często wywołując niepokój u rodziców dzieci.
Cel pracy. Określenie częstości występowania, rodzaju i czynników towarzyszących zmianom błony śluzowej jamy ustnej stwierdzanych u noworodków i niemowląt.
Materiał i metody. Rekrutacja uczestników odbywała się wśród dzieci, które były na wizycie w celu oceny pierwszego ząbkowania. Matki odpowiedziały na pytania dotyczące historii choroby, a dzieci przeszły badanie kliniczne przeprowadzone przez lekarzy pedodontów po przeszkoleniu, kalibracji i badaniu pilotażowym.
Wyniki. W badaniu wzięło udział 248 dzieci w wieku do 12 miesięcy. Średnia liczba zębów (SD) wynosiła 1,96 (2,55). U 1,25% dzieci występowały zęby noworodkowe. Próchnicę zębów stwierdzono u 3,2% dzieci, urazowe uszkodzenia zębów – u 7%, hipoplazję szkliwa – u 1,9%, a zmiany błony śluzowej – u 19,8% pacjentów.
Wnioski. Zmiany w jamie ustnej u niemowląt mogą być związane z ząbkowaniem, zębami i błoną śluzową jamy ustnej. Jedno dziecko na 5 ma zmiany dotyczące błony śluzowej, najczęściej guzki Bohna lub kandydozę. Istnieje znacząca korelacja między częstością występowania zębów noworodkowych a obecnością guzków Bohna.
Summary
Introduction. Oral lesions in newborns and infants represent a wide range of diseases often creating apprehension and anxiety among parents.
Aim. We aimed to assess the type, prevalence and associated factors of oral lesions in newborns and infants.
Material and methods. Participants were recruited during a two-year cross-sectional study among children who had their first teething assessed. The mothers answered medical history questions and the children underwent a clinical examination by paediatric dentists after training, calibration, and pilot study.
Results. Two hundred and forty-eight children aged up to 12 months took part in the study. The mean number of teeth (SD) was 1.96 (2.55). 1.25% of children had natal teeth. Dental caries was observed in 3.2% children; traumatic dental injuries caused by falling in 7%; enamel hypoplasia in 1.9%, and mucosal lesions in 19.8% (Bohn’s nodules, mucocele, candidiasis).
Conclusions. Oral lesions in infants may involve teething, teeth and oral mucosa. One child out of five children has mucosal lesions, most often Bohn’s nodules or candidiasis. There is a significant correlation between the prevalence of natal/neonatal teeth and of Bohn’s nodules.
Introduction
The oral cavity in infants is very specific. Some of its aspects result from physiological characteristics and physical and functional development; others are pathological and require medical interventions (1, 2). Lesions typical for infants were most often caused by teething and included eruptive gingivitis/cysts and traumas (such as Riga-Fede disease and candidiasis). Parents were also worried about early teething, including natal and neonatal teeth (3, 4). Oral lesions prevailed in even 30% of the examined babies (5). That prevalence could result from local, genetic, systemic or environmental factors during pregnancy and after birth (1, 3-6). There were few studies on oral lesions in infants and newborns (1, 4-12). The topic is not often taken up by researchers, so there is a knowledge gap that needs to be filled. The following survey was conducted due to the observation of various changes in the oral cavity among patients reporting for a dental visit.
Aim
The objective of the study was to assess the type, prevalence, and associated factors of oral lesions in newborns and infants.
Material and methods
Participants were recruited in the Department of Paediatric Dentistry, Medical University of Warsaw during a two-year cross-sectional study among children under the age of 12 months. The study was authorised by a Commission for Bioethics of Medical University of Warsaw and has been conducted in full accordance with ethical principles, including the World Medical Association Declaration of Helsinki. Inclusion criteria were as follows: age up to 12 months, residence in Warsaw agglomeration, and written parent consent for participation in the study. Participants were excluded if they had dental and jaw anomalies. Mothers were asked about the medical history and children underwent a dental clinical examination. The medical history included questions about child and parent age, socioeconomic factors (socioeconomic status, education, number of children in the family and cigarette smoking), pregnancy and delivery specifics (pregnancy duration, bad habits, general health problems, delivery type, baby birth weight, complications and feeding), and general health (chronic diseases, medication and eating habits). Participants underwent the clinical examination at the dental clinic according to WHO criteria; the anatomical and physiological oral characteristics of the children were taken into account (13); differential diagnosis was used to assess oral lesions (14). Intraoral examination included mucosa examination and potential lesion type and location. Tongue and upper lip frenal attachments were assessed. Tooth examination included the presence/type of erupted teeth, carious lesions (white spot lesions – WSL, precavitated lesions [d], caries [p]) and traumas. Alveolar ridges were examined for tooth buds and the accompanying symptoms. Clinical examinations were performed by 4 paediatric dentists after calibration (inter- and intraexaminer reliability was assessed by Cohen’s kappa coefficient and in all cases it was above 0.8).
Statistics
The comparison of proportions (percentages) was conducted using Fisher exact test. The analyses were performed in version 12 of Statistica (StatSoft, Tulsa, OK). Significance level was set at 0.05.
Results
Two hundred and forty-eight children under the age of 12 months, including 55.2% of boys, took part in the study. Ninety children (54.4% of boys) were less than six months old; 158 (55.7% of boys) were more than six months old. Table 1 presents the characteristics of the examined children at different ages. The average birth weight (± standard deviation [SD]) equalled 3202.1 (± 674.4) grams. The average pregnancy (± SD) lasted 38.5 (± 3.8) weeks.
Tab. 1. Characteristics of the examined children at different ages
| | Total
(n = 248)
n (%) | Child age (months) |
0-6
n = 90
n (%) | > 6-12
n = 158
n (%) |
| Age in months (mean [SD]) | | 7.38 (2.9) | 4.33 (1.23) | 9.11 (2.0) |
| | | | | |
| | | | | |
| | | | | |
| Birth at < 37 weeks of gestation | | 27 (10.9) | 4 (4.4) | 23 (14.6) |
| | | | | |
| | | | | |
| Complications at birth | oxygen deprivation | 17 (6.9) | 4 (4.4) | 13 (8.2) |
| infection requiring antibiotic therapy | 20 (8.1) | 7 (7.8) | 13 (8.2) |
| | | | | |
| Child chronic diseases | | | | |
| acid reflux | 11 (4.4) | 3 (3.3) | 8 (5.1) |
| malabsorptions | 8 (3.2) | 4 (4.4) | 4 (2.5) |
| congenital anomalies of urinary tract | 2 (0.8) | 0 (0) | 2 (1.3) |
| heart defects | 6 (2.4) | 2 (2.2) | 4 (2.5) |
| calcium and phosphate metabolism disorders | 6 (2.4) | 3 (3.3) | 3 (1.9) |
| anaemia | 2 (0.8) | 1 (1.1) | 1 (0.6) |
| diabetes | 2 (0.8) | 1 (1.1) | 1 (0.6) |
Eight mothers smoked and two mothers consumed alcohol during pregnancy. Ninety-three women (37.5%) reported general health problems during pregnancy as follows:, 32 (34.4%) anaemia, 24 (25.8%) respiratory tract infections, 19 (20.4%) diabetes, and 18 (19.4%) urinary tract infections. Table 2 presents the parent and family characteristics of the examined children.
Tab. 2. Parent and family characteristics of the examined children
| | Mother
n (%) | Father
n (%) |
| Age of parents (in years) | ≤ 25 | 36 (14.5) | 23 (9.5) |
| 26-30 | 75 (30.2) | 57 (23.7) |
| 31-35 | 87 (35.1) | 99 (41.1) |
| 36-40 | 42 (17) | 48 (19.9) |
| > 40 | 8 (3.2) | 14 (5.8) |
| Education | primary school | 7 (2.8) | 4 (1.7) |
| secondary school | 88 (35.5) | 75 (31.1) |
| higher then secondary school | 153 (61.7) | 162 (67.2) |
| Family socio-economic status | low | 4 (1.6) |
| | average | 82 (33.1) |
| | good | 162 (65.3) |
| Number of children in a family (mean [SD]) | 1.7 (0.85) |
| Cigarette smoking at home | 61 (24.6) |
In 39 children (15.7%), five under six months of age and 34 over six months of age, the incisal edge or the cusp of a tooth was possible to detect through the oral mucosa. One child had swollen gums and 16 had red gums around the erupting tooth. Five children over six months of age had an eruption cyst and one child over six months of age presented with an abscess. Table 3 presents the dental status of the examined children and figure 1 presents the type of erupted teeth. Six children (1.25%) had natal teeth, one child (0.4%) had neonatal tooth and one child (0.4%) had a mesiodens.

Fig. 1. Percentage of the type of erupted teeth in the examined infants
Tab. 3. Dental status of the examined children in different age
| | | Children age, months |
| Teeth | Total
n (%) | 0-6
n (%) | > 6-12
n (%) |
| Examined children | 248 (100) | 90 (100) | 158 (100) |
| Children with at least one erupted tooth* | 157 (63.3) | 28 (31.1) | 129 (81.6) |
| Number of erupted teeth in each children (mean [SD]) | 1.96 (2.55) | 0.41 (0.89) | 2.82 (2.79) |
| Traumatic dental injuries** | 11 (7) | 2 (2.2) | 9 (7.6) |
| Dental caries (d + p > 0)** | 5 (3.2) | 0 | 5 (3.9) |
| Enamel hypoplasia** | 3 (1.2) | 0 | 3 (1.9) |
*As a percent of examined children
**As a percent of children with at least one erupted tooth
Mandibular central incisors were generally the first to erupt, followed by maxillary central incisors. Tooth 71 erupted on average (95% confidence interval) at 7.51 (6.96-8.06) months, tooth 81 at 7.97 (7.41-8.52) months, tooth 51 at 9.81 (9.39-10.23) months, and tooth 61 at 10.10 (9.66-10.54) months. Five among the 33 children (15.2%), who had four erupted maxillary incisors, had caries on their labial surfaces. Two children, aged 8.8 and 10.4 months, had only white spot lesions (d = 1 and d = 4, respectively); three children, aged 10.4, 12, and 12 months had caries (p = 4). Eleven maxillary central incisors and two mandibular central incisors in 11 children underwent traumatic injuries, including partial tooth luxation (n = 7) and crown fracture involving both enamel and dentin (n = 2), and tooth luxation (n = 4). Falling caused all traumas (tab. 4).
Tab. 4. Prevalence of various characteristics/lesions of oral mucosa
| Oral mucosa | Total
n (%) | Child age, months | |
0-6
n (%) | > 6-12
n (%) | P |
| | Examined children | 248 (100) | 90 (100) | 158(100) |
| Upper lip frenal attachment |
| mucosal | 25 (10.1) | 13 (14.4) | 12 (7.6) | 0.123 |
| gingival | 88 (35.5) | 27 (30.0) | 61 (38.6) | 0.214 |
| papillary | 83 (33.5) | 31 (34.4) | 52 (32.9) | 0.889 |
| incisive papilla penetrating | 52 (21.0) | 19 (21.1) | 33 (20.9) | 1.000 |
| Short lingual frenulum | 37 (14.9) | 14 (15.5) | 23 (14.6) | 0.832 |
| Mucosal lesions | 49 (19.8) | 24 (26.6) | 25 (15.8) | 0.560 |
| Bohn’s nodules | 24 (9.7) | 10 (11.1) | 14 (8.9) | 0.656 |
| mucocele | 7 (2.8) | 5 (5.6) | 2 (1.3) | 0.103 |
| oral candidiasis | 5 (2) | 5 (5.6) | 0 (0.0) | 0.006* |
| aphthous stomatitis | 4 (1.6) | 1 (1.1) | 3 (1.9) | 1.000 |
| primary oral herpes | 3 (1.2) | 1 (1.1) | 2 (1.3) | 1.000 |
| fibroma | 3 (1.2) | 1(1.1) | 2 (1.3) | 1.000 |
| Epstein’s pearls | 2 (0.8) | 1 (1.1) | 1 (0.6) | 1.000 |
| epulis | 1 (0.4) | 0 (0.0) | 1(0.6) | 1.000 |
p-values are based on Fisher exact test for comparisons of children in various age
*statistical significance at p < 0.05
Table 4 presents the upper lip frenal attachments, the short lingual frenulum, and the lesions of the oral mucosa. The mucosal lesions were observed in 19.8% (Bohn’s nodules, mucocele, candidiasis). Different oral lesions were found in different location as follows: oral mucocele located on the lower lip (n = 5) and on cheek mucosa at the corners of the mouth (n = 2); fibromas located on the lower lip (n = 1) and tongue (n = 2) and epulis (n = 1) located at the incisive papilla. Mucocele (fig. 2) accompanied erupted teeth in five patients; they also prevailed in two children with no teeth. Four generally healthy children (1.8%) and one with malabsorption (3.6%) had candidiasis. Oral herpes and mucocele were seen only in generally healthy children. Signs of candidiasis were observed on cheek mucosa at the corners of the mouth and on the tongue (tab. 4).

Fig. 2. Oral mucocele
The Spearman’s rank correlation coefficient did not indicate any statistically significant correlations between mucosal lesions, socio-economic factors, pregnancy, delivery and feeding. There were two statistically significant correlations: one correlation between oxygen deprivation at birth and traumatic dental injuries (r = 0.202, p = 0.001); and one correlation between the prevalence of natal/neonatal teeth and of Bohn’s nodules (r = 0.134, p = 0.035).
Discussion
In the present study, patients were assessed both before and at teething. Most patients had at least one erupted tooth (most often maxillary/mandibular central incisor). These results confirmed the findings of other Polish studies on deciduous teeth eruption in the past 30 years (15). Teething started in the second or third month of life; the first tooth erupted on average between 6.6 (2.09) (2005) and 6.62 (1985) months up to 7.3 months (1979) (15). International studies confirmed these results; the first tooth erupted on average between 6.9 and 9.2 months (16) and most of the time it was the mandibular lateral incisor. Teething anomalies (natal/neonatal teeth) were also reported in the present study. Natal teeth are present at birth, while neonatal teeth erupt within the first month after birth. The studies established that natal teeth prevailed in 1:700 to 1:30 000 births, which was three times higher than the prevalence of neonatal teeth (2, 17); in the present study these anomalies prevailed in 2.4 and 0.4% of patients respectively, which confirmed those statistics. The extraction of a tooth is recommended if the tooth is supernumerary or if the tooth interferes with breastfeeding or in order to prevent possible aspiration due to tooth mobility. If natal teeth lead to traumatic ulceration of the tongue, known as Riga-Fede disease (fig. 3), they should be treated by smoothing the incisal edge of the tooth (2).
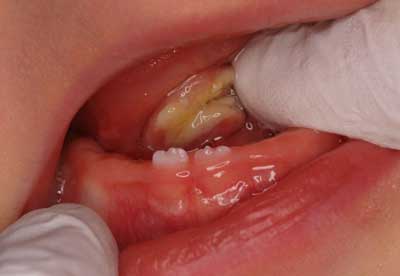
Fig. 3. Riga-Fede disease
The oral mucosa around the erupting teeth was also assessed in the present study. Teething could be accompanied by mucosal lesions caused by systemic or local factors (15, 16, 18). Local lesions included hypersalivation; stomatitis and the related redness, itching and gum swelling; eruption cysts; periodontal abscess; and hematomas (15, 16, 18). Such lesions were also observed in the present patients. Caries prevailing shortly after tooth eruption was alarming. Carious lesions (white spot lesions and cavitated lesions) were localised on the labial surfaces of the freshly erupted maxillary incisors (3.9% of patients with at least one erupted tooth; 15% of patients with all maxillary incisors), a typical location for early childhood caries (ECC). It might be associated with tooth immaturity after eruption, a thinner layer of hard tissues in deciduous teeth, or insufficient saliva protection of maxillary incisors. Saliva wetted them very little and had a low buffering capacity; furthermore, its secretion decreased during sleep (19). The risk of ECC increased with bottle or breast feeding, sweetened drinks just before sleeping or at night, and poor hygiene routines after feeding. Other studies also confirmed the prevalence of caries in children under the age of one, however it was lower (1.7% of patients with cavitated lesions and 7.7% with precavitated lesions) (1, 19, 20).
There were few studies on deciduous teeth trauma in infants. Dental traumas are common when children start walking. Falls and fights are the most common causes of tooth injury in children. Mouth injuries can also occur when children trip or are pushed with an object in the mouth. Castro Brezoo and Dreyer Arroyo (21) indicated that, similarly to the present study, traumas touched very few patients under the age of one (1.88 vs 4.5% in the present study). The Spearman’s rank correlation coefficient indicated a statistically significant positive correlation between oxygen deprivation and dental trauma. The main reason of a newborn hypoxia is a reduced partial pressure of oxygen in blood (hypoxemia) leading to the oxygen deficit in tissues (hypoxia). The perinatal hypoxia is recognized when following criteria are met: metabolic or mixed acidosis (pH < 7.10 or deficiency of alkali < 12 mEq/l in umbilical cord blood) and 0-3 Apgar points above fifth minute of life. The criteria also include neurological complications, for example: hypoxic ischemic encephalopathy, hypotonia, convulsion, coma or multi-organ damage symptoms directly after birth (22). There are three degrees of encephalopathy due to Sarnat’s scale (mild; moderate; severe) (22). Neurological complications (cerebral palsy, epilepsy) often result from moderate or severe stadium and can affect further psychomotor growth of the child (22). In the present study almost 7% of the patients suffered mild oxygen deprivation at birth. Zhu et al. (23) performed a meta-analysis and confirmed a potential correlation between oxygen deprivation at birth and ADHD (attention deficit hyperactivity disorder) in children (excessive activity in children could lead to an increased number of traumas). The association between oxygen deprivation at birth and traumatic dental injuries would require more studies, including a neurological evaluation of the children.
The size and positioning of the frenal and lingual attachments is individual and may evolve (24). In breastfed infants, an abnormal attachment of maxillary frenum is a significant factor contributing to caries formation. This is partially due to the inability of infants to remove residual milk from the area between the lip and labial surfaces of the maxillary incisors (25). An early diagnosis and treatment using Erbium: YAG or diode laser can prevent caries development (25, 26). Most patients in the present study had papillary and incisive papilla penetrating attachments; gingival and mucosal attachments were less frequent, which confirmed the findings of other studies. Around 15% of the patients had a short lingual frenulum, which was higher than in the other studies (2, 25). This type may have different clinical implications, including difficulties with breastfeeding. Such anomalies were easily surgically corrected.
The lesions most often appear in the different studies are: recurrent aphthous stomatitis (0.9-10.8%), labial herpes (0.78-5.2%), fissured tongue (1.49-23%), geographic tongue (0.60-9.8%), oral candidiasis (0.01-37%) and traumatic injury (0.09-22.15%) (8).
One child out of six children had oral lesions (14.9%). George et al. (3) (13.8%) and Baldani et al. (9) (21%) encountered a similar prevalence; Bessa et al. (6) (27%) a higher one. In opposite to the above – mentioned studies, Bezzera and Costa (7) encountered very few patients (2.3%) with mucosal lesions. This varying prevalence of oral mucosal lesions in children was caused by different factors, including social-demographics, dentist knowledge and experience with mucosal diseases, different diagnostic criteria, and the description of few lesions in each survey. Baldani et al. (9) examined infants during routine appointments and indicated a similar lesion prevalence to the one in the present study. They also noted that most lesions prevailed in children aged up to three months (26.98%). In the present study the mucosal lesions prevailed most often in children up to 6 months, however mucocele and candidiasis prevailed statistically significantly more often. The most prevalent condition according to Baldani et al. (9) was the inclusion cyst (35.71%), followed by candidiasis (11.90%). In a similar age group, up to two years of age, Yilmaz et al. (11) detected mucosal lesions in one fifth of their patients (21.27%). The most common lesions were candidiasis (10.70%), Epstein’s pearls (2.68%), and geographic tongue (2.68%). The frequency of children with mucosal alterations was higher in the group of children from two to twelve months, which coincided with the results of the present study.
Oral mucosa cysts are classified according to nature, location, and putative ontogenesis. Gingival hyperkeratotic findings might be compatible with Bohn nodules (small, round, and clustered papules) and dental lamina cysts (large, smooth, hard plaquish lesions). Bohn’s nodules were the most frequent mucosal lesion, prevailing mainly on alveolar ridges in the first weeks of life (3, 27, 28). These were benign single or multiple cysts, that were smooth, yellowish or white, and filled with keratin. Prevalence data are to be considered approximate because of confusion regarding nomenclature and difficulty in spotting and documenting these lesions correctly. Their prevalence in the present study (9.7%) was similar to that in other studies. George et al. (3) observed them in 47.40% of Indian children; Padovani et al. (27) only in 4.1% of children. According to Lewis (28), cysts, including Bohn’s nodules or Epstein’s pearls, could prevail in 65-85% of newborns; they were sometimes confused with natal or neonatal teeth. Although prevalence is high, Bohn’s nodules or Epstein’s pearls are rarely observed by dentists because they disappear within two weeks to five months. In some cases, however, they may remain and surgical opening is necessary. The present study indicated a significant positive correlation between Bohn’s nodules and natal and neonatal teeth, which could suggest different congenital disorders could prevail simultaneously in the same tissues.
According to literature between 0.57 and 15% of infants (3, 5-9, 11, 12, 27) had oral candidiasis. It was promoted by systemic diseases (immunodeficiencies, diabetes, and endocrine disorders), early birth, low birth weight, and long term use of broad-spectrum antibiotics. In the present study, five patients had candidiasis, including one with a systemic disease and four generally healthy ones (3.6 to 1.8% respectively). Padovani et al. (27) detected candidiasis in 6.3% of the babies, without providing details on their general health; Yilmaz et al. (11) detected candidiasis in 10.70% of children aged between 2 and 12 months. Candidiasis prevailed more often in younger children because of the immaturity of their immune system. The neonates can be colonised by Candida albicans due to birth contact with the infected mother (8, 11), or unawareness of their parents (performing poor child oral hygiene) care (4). Candidiasis can be associated with antibiotic therapy and with the use of pacifiers (6).
The results of Xiao et al. study (29) revealed that the presence of oral C. albicans is associated with a highly acidogenic and acid-tolerant bacterial community in S--ECC, with an increased abundance of plaque Streptococcus mutans) and certain Lactobacillus/Scardovia species and salivary/plaque Veillonella and Prevotella, as well as decreased levels of salivary/plaque Actinomyces. Concurrent with this microbial community assembly, the activity of glucosyltransferases (cariogenic virulence factors secreted by S. mutans) in plaque was significantly elevated when C. albicans was present. Children and mothers shared microbiota composition and diversity, suggesting a strong maternal influence on children’s oral microbiota.
Mucocele prevailed in children, but rarely in newborns or infants. According to various studies, between 0.08 and 2.7% (5, 6, 8, 11, 12, 30) of the infants had mucocele, which was confirmed in the present study. There were two types of mucocele: congestive and resulting from salivary duct obstruction; the latter was more common in children. They could also be divided according to their location: on the floor of the mouth (small cysts) or elsewhere (mucocele) (29). These lesions most often prevailed on the lower lip; in infants they most often resulted from mucosal lesions caused by sucking (6). Infants also sucked their fingers and oral mucosa when teething to ease the pain, which meant they injured the salivary duct in the small salivary gland. Salivary duct injuries in infants could also result from finger sucking before birth, coming through the birth canal, or using forceps at delivery. Furthermore, congenital defects of the ducts could obstruct saliva outflow, causing mucocele. Zhi et al. (31) described 11 cases of small cysts in patient with no trauma histories and therefore hinting at congenital causes. In the present study mucocele were detected in seven patients (2.8%), including five under six months, confirming the results of other studies stating that mucocele prevailed more often in newborns than in infants (11, 30).
With regard to the management of the conditions, it was noticed that no treatment was required in most of the cases. These findings were in agreement with those previously reported. The oral mucosa lesions in children are overlooked, misdiagnosed, and inadequately treated (7). Most of them are benign and do not require any treatment (3). The recognition and distinction between normal and altered oral structures is essential. Dentist must be aware of those oral lesions in newborns and infants in order to inform their parents and to treat when necessary.
The present study had some limitations. Lesion detection depended on the experience and diagnosis of the dentist, despite prior training, calibration, and pilot study. Oral lesions were diagnosed after one single examination without neither taking into account the frequency nor the duration, which likely led to an underestimation of the prevalence of the lesions. Despite these limitations, the results provided data on the most important oral mucosal diseases in the paediatric population in terms of prevalence and differential diagnosis of oral lesions in newborns and infants.
Conclusions
Oral lesions in infants may involve teething, teeth and oral mucosa. One child out of five children has mucosal lesions, most often Bohn’s nodules or candidiasis. There is a significant correlation between the prevalence of natal/neonatal teeth and of Bohn’s nodules.
Piśmiennictwo
1. Tomizawa M, Sano T, Noda T: Oral conditions in Japanese infants: a retrospective study. Pediatr Dent J 2007; 17: 65-72.
2. Van Heerden WFP, Van Zyl AW: Diagnosis and management of oral lesions and conditions in the newborn. S Afr Fam Pract 2010; 52: 489-491.
3. George D, Bhat SS, Hegde SK: Oral findings in newborn children in and around Mangalore, Karnataka State, India. Med Princ Pract 2008; 17: 385-389.
4. Patil S, Rao RS, Majumdar B et al.: Oral Lesions in Neonates. Int J Clin Pediatr Dent 2016; 9: 131-138.
5. Majorana A, Bardellini E, Flocchini P et al.: Oral mucosal lesions in children from 0 to 12 years old: ten years’ experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: e13-18.
6. Bessa CNF, Santos PJB, Aguiar MCF et al.: Prevalence of oral mucosal alterations in children from 0 to 12 years old. J Oral Pathol Med 2004; 33: 7-22.
7. Bezzera S, Costa I: Oral conditions in children from birth to 5 years: the findings of a children’s dental program. J Clin Pediatr Dent 2000; 25: 79-81.
8. Rioboo Crespo MR, Planells del Pozo P, Rioboo Garc?a R: Epidemiology of the most common oral diseases in children. Med Oral Patol Cir Bucal 2005; 10: 376-387.
9. Baldani MH, Lopes CM, Scheidt WA: Prevalence of oral changes in children attending public pediatric dental clinics in Ponta Grossa, PR, Brazil. Pesqui Odontol Bras 2001; 15: 302-307.
10. Goyal R, Jadia S, Jain L et al.: A Clinical Study of Oral Mucosal Lesions in Patients Visiting a Tertiary Care Centre in Central India. Indian J Otolaryngol Head Neck Surg 2016; 68: 413-416.
11. Yilmaz AE, Gorpelioglu C, Sarifakioglu E et al.: Prevalence of oral mucosal lesions from birth to two years. Niger J Clin Pract 2011; 14: 349-353.
12. Shulman JD: Prevalence of oral mucosal lesions in children and youths in the USA. Int J Paediatr Dent 2005; 15: 89-97.
13. Walker M: Influence of infants’ anatomy and physiology. [In:] Breastfeeding management for the clinicians: using the evidence 4th ed. Jones and Bartlett Learning, 2016: 131-137.
14. Finkelstein M: A guide to clinical differentia diagnosis of oral mucosal lesions; https://www.dentalcare.com/en-us/professional-education/ce-courses/ce110.
15. Olczak-Kowalczyk D, Boguszewska-Gutenbaum H, Janicha J et al.: Selected issues of baby teething. Nowa Stomatol 2011; 2: 73-76.
16. Li RX, Hu Y: A cross-sectional survey on the patterns of primary teeth eruption in 2 581 children. Zhonghua Er Ke Za Zhi 2017; 55: 37-41.
17. Rao RS, Mathad SV: Natal teeth: Case report and review of literature. J Oral Maxillofac Pathol 2009; 13: 41-46.
18. Olczak-Kowalczyk D, Turska-Szybka A, Gozdowski D et al.: Longitudinal study of symptoms associated with teething: Prevalence and mothers’ practices. Pediatr Pol 2016; 91: 533-540.
19. Anil S, Anand PS: Early Childhood Caries: Prevalence, Risk Factors, and Prevention. Front Pediatr 2017; 5: 157.
20. Tham R, Bowatte G, Dharmage SC et al.: Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr 2015; 104: 62-84.
21. Castro Brezoo PF, Dreyer Arroyo E: Prevalence of dental trauma of infants attended at Dr. Sótero del Río Hospital. Rev Clin Periodoncia Implantol Rehabil Oral 2012; 5: 127-130.
22. Mahar S, Parkash J, Das C et al.: Outcome of term newborns with hypoxic ischemic encephalopathy. Gomal J Med Sci 2017; 15: 42-45.
23. Zhu T, Gan J, Huang J et al.: Association Between Perinatal Hypoxic-Ischemic Conditions and Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis. J Child Neurol 2016; 31: 1235-1244.
24. Boutsi EA, Tatakis DN: Maxillary labial frenum attachment in children. Int J Paediatr Dent 2011; 21: 284-288.
25. Kotlow LA: The influence of the maxillary frenum on the development and pattern of dental caries on anterior teeth in breastfeeding infants: prevention, diagnosis, and treatment. J Hum Lact 2010; 26: 304-308.
26. Segal LM, Stephenson R, Dawes M et al.: Prevalence, diagnosis, and treatment of ankyloglossia: methodologic review. Can Fam Physician 2007; 53: 1027-1033.
27. Padovani MC, Santos MT, Sant’ Anna GR et al.: Prevalence of oral manifestations in soft tissues during early childhood in Brazilian children. Braz Oral Res 2014; 28. pii: S1806-83242014000100246.
28. Lewis DM: Bohn’s nodules, Epstein’s pearls, and gingival cysts of the newborn: a new etiology and classification. J Okla Dent Assoc 2010; 101: 32-33.
29. Xiao J, Grier A, Faustoferri RC et al.: Association between Oral Candida and Bacteriome in Children with Severe ECC. J Dent Res 2018; 97(13): 1468-1476.
30. Shapira M, Akrish S: Mucoceles of the oral cavity in neonates and infants – report of a case and literature review. Pediatr Dermatol 2014; 31: e55-58.
31. Zhi K, Wen Y, Ren W et al.: Management of infant ranula. Int J Pediatr Otorhinolaryngol 2008; 72: 823-826.


