Jolanta Jadczyszyn, *Lidia Zawadzka-Głos
Acute appendicitis in children during the COVID-19 pandemic
Ostre zapalenie wyrostka sutkowego u dzieci w okresie pandemii COVID-19
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Wstęp. Powikłania usznopochodne u dzieci występują rzadko i są nadal stanem bezpośredniego zagrożenia życia. U dzieci najczęściej występującym powikłaniem jest ostre zapalenie wyrostka sutkowego w przebiegu ostrego zapalenia ucha środkowego. Dominującymi objawami są: silny ból ucha i okolicy zausznej, obrzęk okolicy zamałżowinowej z odstawaniem małżowiny usznej i opadaniem tylno-górnej ściany przewodu słuchowego zewnętrznego.
Cel pracy. Celem pracy było przedstawienie pacjentów z ostrym zapaleniem wyrostka sutkowego w okresie pandemii COVID-19 i ocena współwystępowania zakażenia wirusem SARS-CoV-2.
Materiał i metody. Przeprowadzono retrospektywną analizę hospitalizowanych pacjentów z ostrym zapaleniem wyrostka sutkowego w latach 2020-2021. Ocenie poddano: współwystępowanie zakażenia SARS-CoV-2 u dzieci z powikłaniem usznopochodnym, sezonowość występowania powikłań, wiek, płeć, metody diagnostyczne i analizę patogenów odpowiedzialnych za rozwój powikłania.
Wyniki. W latach 2020-2021 w Dziecięcym Szpitalu Klinicznym Uniwersyteckiego Centrum Klinicznego Warszawskiego Uniwersytetu Medycznego hospitalizowano 23 pacjentów z ostrym zapaleniem wyrostka sutkowego. U jednego pacjenta stwierdzono współistniejące zakażenie SARS-CoV-2. W analizowanej grupie obserwuje się znaczną przewagę chłopców nad dziewczynkami M:K = 2,8:1. Średni wiek pacjentów wynosił 5 lat. U wszystkich pacjentów stwierdzono objawy kliniczne ostrego zapalenia wyrostka sutkowego w postaci obrzęku tkanek miękkich okolicy zausznej ze stanem zapalnym skóry i odstawaniem małżowiny usznej. W badaniu otoskopowym stwierdzono u 22% dzieci ropny wyciek z ucha środkowego, zaś u pozostałych 78% nie stwierdzono perforacji błony bębenkowej, u których ropna wydzielina zalegała w całej jamie bębenkowej. W uzyskanych badaniach bakteriologicznych u 74% dzieci nie stwierdzono patogenów chorobotwórczych. Wszyscy pacjenci mieli zastosowaną dożylną antybiotykoterapię i leczenie operacyjne.
Wnioski. Wśród hospitalizowanych pacjentów z ostrym zapaleniem wyrostka sutkowego największą zachorowalność obserwowano w okresie styczeń-kwiecień 2020 roku oraz wrzesień-grudzień 2021 roku. Od maja do września 2020 roku nie hospitalizowano żadnego pacjenta z powikłaniem usznopochodnym. Był to okres, kiedy od maja 2020 roku żłobki, przedszkola i szkoły zaczęły podlegać ścisłym zaleceniom Głównego Inspektoratu Sanitarnego w zakresie reżimu sanitarnego, co mogło się przyczynić do zmniejszonej zachorowalności na infekcje górnych dróg oddechowych. Hospitalizacja pacjenta z ostrym zapaleniem wyrostka sutkowego ze współistniejącym powikłaniem zakażenia SARS-CoV-2 wymagała izolacji pacjenta na oddziale obserwacyjno-izolacyjnym naszego szpitala. Jednak nie obserwowano u pacjenta zaburzeń oddechowych i krążeniowych, nie wymagał intensywnej opieki medycznej.
Summary
Introduction. Ear complications in children are rare and are still an immediate life threatening condition. In children the most common complication is acute mastoiditis in the course of acute otitis media. The predominant symptoms are severe pain in the ear and the retroauricular region, swelling of the muzzle region with protrusion of the auricle and subsidence of the posteriori-top wall of the external auditory canal.
Aim. The aim of this study was to present patients with acute mastoiditis during the COVID-19 pandemic and to evaluate the co-infection with SARS-CoV-2 virus.
Material and methods. A retrospective analysis of hospitalized patients with acute mastoiditis between 2020 and 2021 was performed. We evaluated the co-occurrence of SARS-CoV-2 infection in children with ear complications, seasonality of complications, age, gender, diagnostic methods and analysis of pathogens responsible for the development of the complication.
Results. Between 2020 and 2021, 23 patients with acute mastoiditis were hospitalized at the Department of Pediatric Otolaryngology University Clinical Center Medical University of Warsaw. One patient was diagnosed with coexisting SARS-CoV-2 infection. In the analyzed group there was a significant predominance of boys over girls M:K = 2.8:1. The mean age of the patients was 5 years. All patients showed clinical signs of acute mastoiditis in the form of swelling of the soft tissues of the behind-the-ear region with inflammation of the skin and withdrawal of the auricle. Otoscopic examination revealed purulent middle ear leakage in 22% of children, while the remaining 78% showed no perforation of the tympanic membrane, with purulent discharge deposited throughout the tympanic cavity. In the bacteriological studies obtained, no pathogens were found in 74% of the children. All patients had intravenous antibiotic therapy and surgical treatment.
Conclusions. Among hospitalized patients with acute mastoiditis, the highest morbidity was observed in January-April 2020 and September-December 2021. No patient with ear complication was hospitalized from May 2020 to September 2020. This was the period when, from May 2020, day nurseries, kindergartens and schools began to be subject to strict sanitary regime recommendations by the Chief Sanitary Inspectorate, which could contribute to a reduced incidence of upper respiratory tract infections. Hospitalization of a patient with acute mastoiditis with a coexisting complication of SARS-CoV-2 infection required isolation of the patient in the observation-isolation unit of our hospital. However, the patient’s respiratory and circulatory distress wasn’t observed and he didn’t require intensive medical care.
Introduction
Acute mastoiditis in children is the most common complication of acute otitis media. Ear complications are much more common in children than in adults and we can divide them into intratympanic and intracranial complications (tab. 1) (1). Mastoiditis is an acute inflammatory condition involving mastoid cells with features of osteitis, developing suddenly with local and general signs of inflammation. The causes of the development of complications of acute otitis media are not clear. It is believed that the course of acute otitis media can be significantly aggravated by pathogen resistance to treatment, ineffective antibiotic therapy, or immunologic disturbances in children (1, 2). In addition, impaired communication between the mastoid cavity and the tympanic cavity and failure to create a perforation of the tympanic membrane with leakage of purulent contents from the middle ear is also a cause of the spread of inflammation beyond the middle ear structures (3). This dynamic process of acute inflammation can destroy the mucoperiosteum and lead by contact, by continuity or by the blood-borne route to the development of complications.
Tab. 1. Classification of ear complications
| Intratemporal complications | Intracranial complications |
| Mastoiditis | Ear meningitis and encephalitis |
| Facial nerve palsy | Thrombophlebitis of the sigmoid sinus |
| Labyrinthitis | Epidural abscess
Subdural abscess
Brain abscess |
| Perilymphatic fistula | Hydrocephalus of the ear |
| Inflammation of the temporal bone pyramid | Ear sepsis |
Aim
The aim of the study was to analyze hospitalized patients with acute mastoiditis with special regard to the etiology of infection and SARS-CoV-2 co-infection. The clinical course, abnormalities in laboratory and additional examinations as well as radiological and histopathological picture analysis were performed.
Material and methods
In 2020-2021, 23 patients with acute mastoiditis were hospitalized at the Department of Pediatric Otolaryngology University Medical of Warsaw during the COVID-19 pandemic. All patients were admitted in the emergency room after an ENT consultation with symptoms of acute mastoiditis and all were tested for SARS-CoV-2 infection. Patients with acute otitis media without clinical signs of developing a complication and patients with chronic otitis media were excluded from the study. The age and gender of patients, etiology of inflammation, location of inflammatory lesions, results of laboratory tests and co-morbidities were evaluated. Additional investigations included computed tomography. All patients were treated with combined intravenous antibiotic therapy with surgery.
Results
The reason for hospital admission of all 23 patients (6 girls and 17 boys) was soft tissue swelling and inflammation of the skin of the behind-the-ear region with auricular protrusion and severe pain in the ear and mastoid region on palpation (fig. 1). The age of the study group of patients is shown in table 2. Eight children (35%) had bilateral otitis media and the remaining 15 patients (65%) had unilateral otitis media with coexisting ear complications. Among the study group, 48% of children developed right-sided mastoiditis and 52% of children developed left-sided mastoiditis. Perforation of the tympanic membrane with leakage of purulent contents from the middle ear on the side of the complication was found in 5 patients. Among general symptoms, fever (70% of children) and cervical lymphadenopathy (4.5%) were the most common. Two patients had symptoms of headache, vomiting, double vision, and paresis of the abducent nerve (VI nerve) in the course of coexisting temporal pyramidal inflammation. 1 boy presented with the development of an intracranial complication in the form of right cavernous sinus thrombosis and was followed by a multispecialty neurologic, hematologic and ophthalmologic consultation. Among the study group, 1 patient had a third diagnosis of acute mastoiditis on the left side after oncological treatment for a pineal tumor and bilateral mild sensorineural hearing loss. Otoscopic examination revealed pressure soreness of the posterior wall of the external auditory canal, thickened, congested and protruded tympanic membrane with purulent discharge throughout the tympanic cavity in 18 patients (78%) (fig. 2). Laboratory results are shown in table 3 finding elevated C-reactive protein (CRP) in 20 patients (87%) with concomitant leukocytosis in 15 patients (65%). The prothrombin time (INR) and the koalin-kephalin time (APTT) were within normal laboratory limits in most patients. Thrombocythemia was present in 13 patients. One patient with acute mastoiditis, severe neuroinfection and positive meningeal signs developed meningitis with pneumococcal sepsis in whom the diagnosis was made on the basis of cerebrospinal fluid (PMR) findings (tab. 4). Cerebrospinal fluid examination and blood culture revealed genetic material of Streptococcus pneumoniae etiology. Microbiological results of intraoperative sampling showed no aerobic or anaerobic bacteria in 70% of patients, 8.6% of children had Streptococcus pneumoniae infection that did not demonstrate antibiotic resistance, and 8.6% of patients were diagnosed with Streptococcus pyogenes. One patient with intraoperatively diagnosed cholesteatoma of the mastoid process had multiple pathogens of Corynebacterium amycolatum. The other pathogens were isolated cases of Staphylococcus aureus, Haemophilus influenzae, and from cultures from multiplication of Streptococcus constellatus or Streptococcus oralis. Complementary tests included temporal bone radiographs, which were performed in 92% of hospitalized patients. All temporal bone CT scans performed showed apneumatic mastoid cells (92% of patients) with erosion of bone septum (36% of children). Among 25% of patients, a subperiosteal abscess was diagnosed, 40% had erosion of the cortical layer of the mastoid process, 20% had thinning of the inner lamina with erosion around the periosteal cells, and 10% of patients had thinning of the area around the lid of the tympanic cavity. A CT scan of the left temporal bone in a patient with coexisting SARS-CoV-2 infection showed a completely a pneumatic tympanic cavity and mastoid cells with features of bony septal erosion and bony erosion of the middle cranial fossa restriction with a normally preserved auditory ossicular chain (fig. 3, 4). The treatment of choice was intravenous antibiotic therapy Cephalosporin III generation with Clindamycin or Metronidazole. Surgical treatment was performed in all cases, in 17 patients an antromastoidectomy with ventilation drainage was performed finding normal preserved ossicles and lateral semicircular canal. Ventilator drainage was performed in 5 children because of their overall good clinical condition, discrete local symptoms, and lack of bone erosion on radiographs. On the other hand, in 1 patient with acute mastoiditis and subperiosteal abscess, cholesteatoma masses were found intraoperatively with complete erosion of the mastoid cells, malleus head and incus, as well as a significant bony defect within the tympanic mastoid cavity lids giving a wide connection to the middle cranial fossa and cholesteatoma masses entering the cranial cavity (fig. 5). She was treated tympanoplasty with reconstruction of the tympanic membrane, ossicular bone and plasticity of the middle cranial fossa. Histopathological findings included inflammatory granuloma with areas of necrosis and perivascular inflammatory infiltrates (16 patients), keratinized squamous epithelium = cholesteatoma (1 patient) and multiple histiocytes (CD68 PGM1) with foam-like cytoplasm and areas of necrosis in the vessel wall with suspected granulomatosis with vasculitis (1 patient with pneumococcal sepsis). All patients with acute mastoiditis had nasopharyngeal swabs performed for SARS-CoV-2 infection by RT-PCR. Only 1 patient (B.G.) with acute mastoiditis had the N2 gene (ct 21.5) and E gene (ct 19.2) of SARS-CoV-2 virus detected. His clinical picture, laboratory findings and imaging studies were not significantly different from those of other patients without SARS-CoV-2 infection. Hospitalization of a patient with acute mastoiditis and coexisting SARS-CoV-2 infection required isolation of the patient in the observation-isolation unit of our hospital. However, the patient wasn’t observed to have respiratory or circulatory distress and didn’t require intensive medical care.

Fig. 1. Clinical picture of acute mastoiditis on the left side
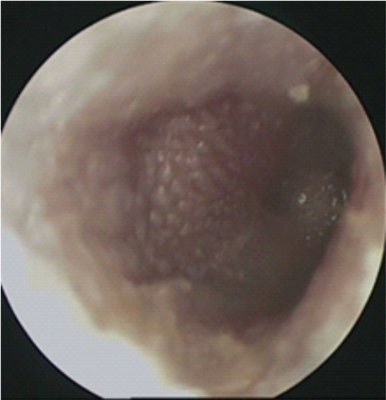
Fig. 2. Picture of the tympanic membrane in acute mastoiditis
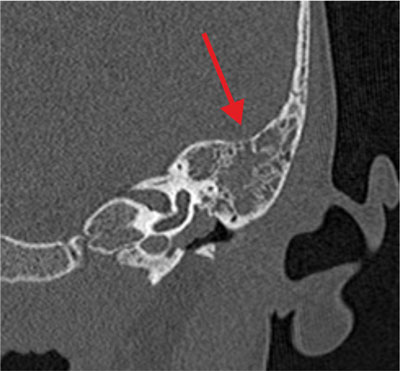
Fig. 3. Picture of mastoiditis (arrow) on temporal bone CT scan of patient with SARS-CoV-2 infection
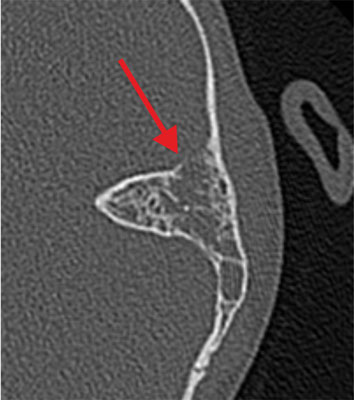
Fig. 4. Temporal bone CT scan – erosion of the lamina propria of the mastoid cavity (indicated by arrow) in patient with SARS-CoV-2 infection

Fig. 5. CT image of the temporal bone in a patient with cholesteatoma – visual erosion of the tympanic and mastoid cavity mastoid (indicated by arrow) and cholesteatoma mass penetration into the middle cranial fossa
Tab. 2. Age of patients
| Patient age interval | Number of patients |
| 0 to 1 years old | 1 patient |
| 1 to 2 years old | 4 patients |
| 2 to 5 years old | 10 patients |
| Over 5 years old | 8 patients |
Tab. 3. Laboratory tests of patients
| Patient | WBC 4-13 thous./uL | Lymph % 24.7-56.0 | Neut % 43-65 | Mono % 4.1-10.9 | RBC 4.2-5.4 mln/uL | HGB 12-16 g/dl | HCT 37-47% | PLT 150-400 thous./uL | CRP 0-1.0 mg/dl | INR 0.9-1.2 | APTT 26-37 sec |
| B.K. | 8.54 | 20.1 | 70.4 | 9.4 | 4.27 | 12 | 34.5 | 264 | 5.9 | 1.07 | 36.35 |
| W.S. | 13.99 | 23.9 | 66.3 | 8.4 | 4.59 | 11.4 | 33.9 | 364 | 3.5 | 1.07 | 34.30 |
| F.M. | 20.29 | 29.8 | 62 | 6.1 | 4.35 | 11.1 | 32.8 | 477 | 3.1 | 1.07 | 27.69 |
| W.I. | 13.49 | 27.7 | 62.1 | 8.5 | 4.22 | 10.3 | 32.2 | 497 | 6.7 | 1.14 | 28.17 |
| F.F. | 14.68 | 22.9 | 68.8 | 7.7 | 3.83 | 10.3 | 30.7 | 404 | 7.6 | 0.94 | 27.26 |
| M.K. | 7.75 | 25.7 | 60.8 | 8.5 | 4.53 | 12.2 | 35.4 | 364 | 0.8 | 1.06 | 24.55 |
| J.K. | 20.92 | 45.0 | 41.6 | 11.2 | 4.49 | 11.3 | 33.6 | 698 | 13.2 | 0.95 | 25.58 |
| A.R. | 10.43 | 38.4 | 52.7 | 7.0 | 4.42 | 11.6 | 33.8 | 515 | 0.7 | 0.97 | 30.78 |
| M.B. | 10.30 | 24.9 | 65.5 | 7.8 | 4.38 | 11.5 | 34.1 | 429 | 1.5 | 1.15 | 28.96 |
| J.N. | 13.04 | 26.4 | 64.8 | 7.4 | 3.93 | 10.8 | 32.0 | 450 | 1.0 | 1.09 | 34.41 |
| S.M. | 23.77 | 15.5 | 74.0 | 9.0 | 4.0 | 10.4 | 31.9 | 326 | 16.4 | 0.96 | 29.16 |
| K.C. | 24.48 | 16.0 | 74.8 | 7.7 | 4.72 | 11.3 | 34.7 | 648 | 4.7 | 1.16 | 27.33 |
| J.R. | 11.81 | 16.4 | 69.7 | 12.5 | 3.97 | 10.6 | 31 | 329 | 14.4 | 0.96 | 32.35 |
| F.B. | 11.08 | 5.3 | 88.7 | 4.9 | 4.59 | 9.3 | 29.4 | 167 | 33.6 | 1.34 | 30.80 |
| P.J. | 15.23 | 30.9 | 62.3 | 5.1 | 4.0 | 10.5 | 31.2 | 723 | 3.42 | 1.09 | 26.04 |
| O.B. | 22.73 | 39.1 | 46.7 | 11.0 | 4.45 | 11.6 | 34.0 | 340 | 9.06 | 0.93 | 32.91 |
| M.F. | 19.89 | 30.6 | 62.4 | 6.0 | 4.5 | 11.6 | 34.3 | 473 | 8.36 | 1.09 | 32.93 |
| H.S. | 16.07 | 39.0 | 49.1 | 10.8 | 3.79 | 10.0 | 30.4 | 509 | 10.19 | 1.04 | 40.90 |
| J.K. | 9.10 | 68.1 | 16.2 | 12.1 | 4.57 | 11.8 | 34.3 | 258 | 9.49 | 0.93 | 34.50 |
| K.W. | 14.65 | 37.1 | 50.2 | 10.9 | 4.56 | 12.0 | 34.8 | 501 | 10.13 | 1.0 | 31.77 |
| M.K. | 14.92 | 35.7 | 48.6 | 12.7 | 3.65 | 10.4 | 29.8 | 390 | 9.34 | 1.03 | 38.5 |
| O.S. | 8.47 | 48.5 | 38.1 | 9.9 | 3.40 | 9.3 | 27.1 | 644 | 3.48 | 1.05 | 28.78 |
| B.G. | 14.33 | 27.5 | 61.8 | 10.2 | 4.20 | 10.7 | 32.4 | 215 | 8.32 | 1.11 | 37.47 |
Tab. 4. Cerebrospinal fluid findings in a patient with meningitis
| Cerebrospinal fluid – normal parameters | Cerebrospinal fluid – results of a patient with meningitis |
| Color watery | Xantochromic |
| Transparence clear | Opalescent |
| Cytosis 0-20/ul | 1676 cells/ul |
| Reaction Nonne-Appelta negative | Positive ++ |
| Reaction Pandy negative | Positive ++ |
| Total protein 15-45 mg/dl | 285.70 mg/dl |
| Glucose 40-85 mg/dl | 5.6 mg/dl |
| Chlorides 115-130 mmol/L | 121 mmol/L |
| Lactic acid 0.5-2.2 mmol/L | 11.2 mmol/L |
Discussion
Since March 2020, a pandemic of SARS-CoV-2 virus infection has started in Poland (4). The viruses belong to the spherical enveloped RNA viruses of the beta-coronavirus subtype and as of 2019, 6 viruses from this group have been recognized to cause human infections (5). Four of them (229E, OC43, NL63, HKU1) cause mild infections and two (SARS-CoV and MERS-CoV) can lead to life-threatening conditions in the form of acute respiratory distress syndromes (5, 6). A new coronavirus that causes COVID-19 acute respiratory disease has been named SARS-CoV-2 by the International Committee on Taxonomy of Viruses (6). Children are a group of patients in whom acute inflammatory conditions of the upper respiratory tract are among the most common. As a result, children are much more likely than adults to develop complications, conditions that are directly life-threatening. Among hospitalized patients with acute mastoiditis, the highest incidence was observed from January to April 2020 and September to December 2021. From May 2020 to September 2020, we didn’t hospitalize any patient with an ear complication (fig. 6). This was the period of reduced incidence of upper respiratory tract infections. It was also the period when day nurseries, kindergartens and schools were subject to strict sanitary regime recommendations of the Chief Sanitary Inspectorate, which might have contributed to reduced incidence of the disease. During the pandemic, every patient admitted to the Children’s Clinical Hospital UCC MUW had a diagnostic test for SARS-CoV-2 virus. The sample was swabbed from the nasopharynx or middle throat, and the test was performed by RT-PCR (reverse transcription polymerase chain reaction, real-time polymerase chain reaction). Molecular testing to detect infection has high specificity but can have low sensitivity (42-71%). False negative results may be caused by too early a sampling time for testing associated with low virus abundance, limited infection to the lower respiratory tract, improper collection, processing, and transport of material (7, 8). During the pandemic period, 23 patients were hospitalized with acute mastoiditis, of which only one patient had a concurrent SARS-CoV-2 infection. Detailed epidemiologic data and available information indicate that SARS-CoV-2 incidence in the pediatric population is less frequent and milder than in adults. Data from China on 45,000 confirmed cases of COVID-19 show that patients under 18 years of age accounted for approximately 2% of this group, including < 1% in children < 10 years of age (9). Similarly, data from Italy indicate a lower rate of SARS-CoV-2 infection in children, that is, of 22,000 COVID-19 cases, only 1.2% were patients < 18 years of age (10). The most commonly reported symptoms were fever and cough, runny nose, fatigue, headache, and diarrhea (11). However, there is a lack of data in the literature regarding hospitalization of patients with acute mastoiditis and SARS-CoV-2 infection. From our previous observations of hospitalized patients with acute mastoiditis, the common pathogen responsible for the occurrence of ear complication is Streptococcus pneumoniae (12). In the hospitalized patient, no bacteria were found in the mastoid material on microbiological examination. This may have been due to the antibiotic therapy used prior to hospitalization in the hospital. In addition, the patient was vaccinated according to the vaccination calendar, including a complete pneumococcal schedule. Currently, there are no data supporting the coexistence of SARS-CoV-2 and pneumococcal infections, but such an interaction is possible (13). It is very important to detect SARS-CoV-2 infection as early as possible, as these children should be hospitalized and isolated in units designed for this purpose. The epidemic has created many epidemiological difficulties that we are constantly facing. During hospitalization of a patient with SARS-CoV-2 infection, the medical and nursing team must wear a barrier gown, gloves, protective goggles, and an N95, FFP2, or FFP3 mask. In COVID-19 infections, symptomatic treatment consists of adequate hydration, adequate caloric intake and if necessary, oxygen therapy to prevent the onset of acute respiratory failure (14-16). There is still no certain evidence for the efficacy of specific causal treatments-antivirals and drugs that affect the inflammatory response resulting from SARS-CoV-2 infection (17). In patients with a complication of acute otitis media, we implement broad antibiotic therapy in the form of third generation Cephalosporins along with an antibiotic that covers the flora of anaerobic pathogens. Infection should not be a barrier to hospitalization and choice of therapy. The need for surgery is associated with adequate staff security. Moreover, there are currently no reliable data for the use of concomitant antiviral therapy in children (18). From previous observations and publications, it is suggested that children are often diagnosed with asymptomatic or subclinical infection that contributes to SARS-CoV-2 virus transmission. Therefore, vaccines are now recognized as an important part of pandemic preparedness (19, 20). For the last 2 years, the whole world has been struggling with new recommendations, constantly new guidelines. However, we are still learning how the SARS-CoV-2 virus spreads, its course, clinical presentation and treatment, and the proper management of complications that occur.
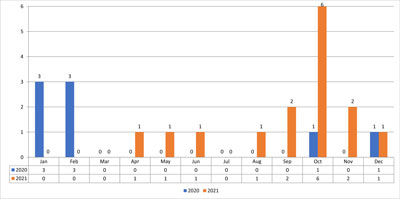
Fig. 6. Showing hospitalized patients in 2020-2021
Piśmiennictwo
1. Goździk-Żołnierkiewicz T: Usznopochodne powikłania wewnątrzskroniowe i wewnątrzczaszkowe. [W:] Janczewski G (red.): Otorynolaryngologia praktyczna. Via Medica, Gdańsk 2005: 122-132.
2. Luntz M, Brods A, Nusem S et al.: Acute mastoiditis ? the antibiotic era, a multicenter study. Int J Pediatr Otorhinolaryngol 2001; 1-9.
3. Nadal D, Herrmann P, Baumann A, Fanconi A: Acute mastoiditis: clinical, microbiological and terapeutic aspects. Eur J Pediatr 1990; 149(8): 560-564.
4. Ministerstwo Zdrowia: www.gov.pl/web/zdrowie/pierwszy-przypadek-koronawirusa-w-polsce.
5. Song Z, Xu Y, Bao L et al.: From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019; 11: 59.
6. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses: The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiology 2020; 5: 536-544.
7. Li Y, Guo F, Cao Y et al.: Insight into COVID-2019 for pediatricians. Pediatr Pulmonol 2020; 55(5): E1-E4.
8. Hao Q, Wu H, Wang Q: Difficulties in False Negative Diagnosis of Coronavirus Disease 2019: A Case Report. 2020; doi:10.21203/rs.3.rs-17319/v1.
9. Wu Z, McGoogan JM: Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020; 323(13): 1239-1242.
10. Livingston E, Bucher K: Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020; 323(14): 1335.
11. Ludvigsson JF: Systemic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109(6): 1088-1095.
12. Jadczyszyn J, Zawadzka-Głos L, Dębska M: Meningitis and encephalitis of the etiology of Streptococcus pneumoniae – a case report. New Medicine 2013; (4): 126-128.
13. Xia W, Shao J, Guo Y et al.: Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 2020; 55(5): 1169-1174.
14. Chiotos K, Hayes M, Kimberlin DW et al.: Multicenter initial guidance of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc 2020; 9(6): 701-715.
15. Jackowska T, Marczyńska M: Procedura postępowania z dzieckiem na SOR/IPP, oddziale pediatrycznym z podejrzeniem lub rozpoznaniem COVID-19. Przegl Pediatr 2020; 49: 1-13.
16. Kuchar E, Karłowicz-Bodalska K: Stosowanie ibuprofenu w czasie pandemii SARS-CoV-2 – co warto wiedzieć? Standardy Medyczne/Pediatria 2020; 17: 276-281.
17. https://covid19treatmentguidelines.nih.gov.
18. Holshue ML, DeBolt C, Lindquist S et al.: First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020; 382: 929.
19. Zhou F, Yu T, Du R et al.: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancelet 2020; 395(10229):1054-1062.
20. http://www.euro.who.int/__data/assets/pdf_file/0004/433813/Guidance-routine-immunization-services-COVID-19-pandemic.pdf.





