© Borgis - New Medicine 3/2012, s. 68-73
*Paweł Kowalczyk
The influence of exocyclic DNA adducts in bacterial and mammalian genome instability
Interdisciplinary Centre for Mathematical and Computational Modelling Warsaw University
Head of Department: prof. Marek Niezgódka, MD, PhD
Summary
Oxidative stress enhances lipid peroxidation (LPO) implicated in the promotion and progression of carcinogenesis. One of the major LPO products is trans-4-hydroxy-2-nonenal (HNE), may to react with guanosine and under peroxidizing conditions also with adenosine. Additionally the same effect may induce environmental carcinogens, e.g. vinyl chloride and its metabolite chloroacetaldehyde (CAA). These compounds CAA and HNE introduce promutagenic exocyclic etheno and propano adducts into DNA, among them 1,N2-propanodeoxyguanine (PdG), 1,N6-ethenoadenine (1,N6-εA), 3,N4-ethenocytosine (3,N4-εC), N2, 3-ethenoguanine (N2,3-εG) and 1,N2-ethenoguanine (1,N2-εG). CAA-induced additionally DNA damage in regions which revealed secondary structure perturbations rich in mutation hot-spots also in bacterial and mammalian genome. These perturbations may inhibited DNA synthesis and induced mechanisms of DNA repair such as BER or NER. Base excision repair constitutes the primary defense against lesions that do not heavily distort the DNA structure. BER is responsible for the removal of a variety of lesions. These include spontaneous hydrolytic depurination of DNA, deamination of bases, products of reaction with hydroxyl radicals, and covalent DNA adducts formed by intracellular LPO and small reactive metabolites, such as methylating agents. Repair is initiated by the action of a damage-specific DNA N-glycosylase that is responsible for the recognition and removal of an altered base through cleavage of the N-glycosylic bond and action of AP-endonuclease. Nucleotide excision repair (NER) is the most versatile and flexible DNA repair pathway of living cells as it deals with a wide range of structurally unrelated DNA lesions. NER corrects a wide array of DNA lesions that distort the DNA double helix, interfere in base pairing and block DNA duplication and transcription. The most common examples of these lesions are the cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts (6-4 PPs) induced by ultraviolet radiation (UV) and bases with large substitutes derived from chemicals such as polycyclic aromatic hydrocarbons or exocyclic adducts.
Formation of exocyclic DNA adducts
Cellular DNA is continuously exposed to a variety of agents that alter its structure. These agents are both endogenous and exogenous, and include normal cellular metabolism, cell injury, inflammation, ionizing radiation and chemical agents. Accumulating evidence indicates that water, oxygen and endogenous alkylation are the main contributors to overall DNA damage (1).
These agents bring a considerable threat to living cells. Although both prokaryotic and eukaryotic cells are equipped with diverse DNA repair systems (2), removal of DNA lesions in an error-free way sometimes is not efficient enough and damage escapes processing before replication. Unrepaired DNA damage leads to various biological consequences, such as mutations or cell death, and subsequently to carcinogenesis, aging, and degenerative diseases (3).
Exocyclic DNA adducts are produced by endogenous and exogenous agents. Of the exocyclic DNA adducts, etheno (ε) bases have been the most widely studied over the last 25 years, as they are is formed by many genotoxic carcinogens, e.g., vinyl chloride or chloroacetaldehyde (4) and are also produced endogenously in animals and man. This class of DNA lesions affects normal Watson-Crick base pairing in DNA and was shown to be mutagenic in E.coli and mammalian cells (4).
It has been estimated that chronic inflammation is involved in the development of about one-forth of all cancers worldwide. Inflammatory response leads to recruitment of activated leukocytes, which release high quantities of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide. Hydrogen peroxide can produce hydroxyl radicals in reaction with metal ions. Direct proof comes from the work of Dizdaroglu (5) who showed that exposure of human cells to activated leukocytes causes DNA base modifications typical of hydroxyl radical attack. ROS also interact with membrane lipids causing their fragmentation and production of reactive aldehydes, which are able to interact with nucleic acids and form exocyclic DNA adducts. Etheno bases were first described by Kochetkov (6), who identified them as fluorescent analogues for biochemical studies and probes for nucleic acid structures although, among different exocyclic adducts only 1,N6 – ethenoadenine possesses fluorescent properties. The renewed interest in exocyclic DNA lesions in the 1990s was due to the development of ultrasensitive detection methods notably for etheno- and propano-DNA adducts which made it possible to study the formation of exocyclic adducts in experimental animals and humans. In 1994, unequivocal identification of the malondialdehyde-derived deoxyguanosine (M1-dG) adduct was reported by Chaudhary (7) in human liver. The same adduct was later also found in human breast and leukocytes. In 1995, Swenberg and co-workers found background levels of etheno- and propano-adducts in DNA of various human and rodent tissues and confirmed the presence of N2, 3-εdG in human liver by mass spectrometric techniques. These findings suggested an endogenous pathway (fig. 1) for the formation of exocyclic adducts via lipid peroxidation products.
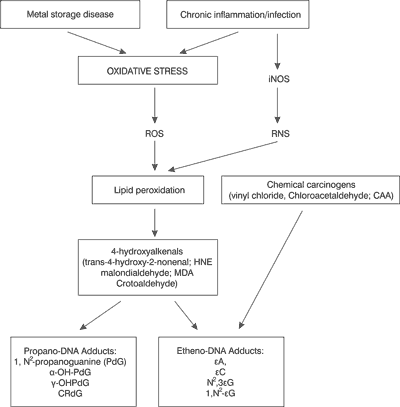
Fig.1. Proposed scheme of carcinogenic factor leading to oxidative stress-induced reactive oxygen species (ROS) and nitrogen (RNS) species; which cause exocyclic DNA-base damage. Where iNOS; inducible nitric oxide synthase (7).
Oxidative stress and lipid peroxidation
Chronic inflammatory infection is one of the sources of free oxygen radicals and also leads to nitric oxide synthase (NOS) induction and therefore to NO synthesis. Oxidative stress processes enhance the generation of such reactive oxygen species as O2, H2O2 and OH.
The most reactive molecule is the hydroxyl radical. Its production can be increased in response to accumulation of free Cu and Fe ions in tissues (mainly in the liver) which is known to occur in some procancerogenic diseases, Wilson disease and primary hemochromatosis. These transient metal ions participate in Fenton and Haber-Weiss reactions to produce hydroxyl radicals:
Fe 2+ + H2O2 → •OH + OH– + Fe3+
O2•– + Fe3+ → O2 + Fe2+
and Haber Weiss reactions;
Fe2+/Fe3+
O2•– + H2O2 → OH• + OH– + O2
Poly-unsaturated lipids, components of lipids bilayers which surround various subcellular micro-environments, are one of the possible targets of free radical attack. The toxic effect of lipid peroxidation (LPO) is connected with the loss of cell membranes function and cell viability (9). Lipid peroxidation occurs in three steps; initiation, propagation and termination, and yields stable products which can either directly react with nucleic acids, or be further metabolized into more reactive compounds (tab. 1).
Table 1. Main carbonyl products of lipid peroxidation separated and stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions (10).
| Polar carbonyls: |
Malondialdehyde
Acrolein
Crotonaldehyde |
| Non-polar carbonyls: |
Hydroxyalkenals:
4-hydroxy-2-hexenal
4-hydroxy-2-nonenal
4-hydroxy-2,5-dienal |
HNE – a major LPO product of divergent reactivity
The major hydroxyalkenal is trans-4-hydroxy-2-nonenal (HNE). Its concentration in human plasma and tissues ranges between 0.1-3.0 μM and can increase to 10 μM in conditions of oxidative stress. HNE is genotoxic in bacterial and mammalian cells. At physiological concentrations, HNE increases the frequency of micronuclei, chromosomal aberrations, sister-chromatid exchanges and point mutations in mammalian cells. HNE is also a potent inducer of the SOS response in Escherichia coli at very low concentrations (0.1-1 μM) (11). Moreover, it exerts a clastogenic effect in human cells, possibly via inactivation of functional SH groups in DNA polymerases. HNE also participates in the regulation of many pathophysiological processes, such as inflammation, cell differentiation, apoptosis, liver fibrosis and carcinogenesis, by formation of adducts in reaction with cellular phospholipids, proteins and nucleic acids. Generation of HNE from low-density lipoprotein and its subsequent binding to apolipoprotein AI and AII, apolipoprotein B and apolipoprotein E (12), has been implicated in the pathogenesis of atherosclerosis (13). Other various adverse biological effects of HNE include inhibition of RNA and DNA synthesis stimulation of neutrophil migration, enzyme inhibition, activation of stress-signaling pathways via transcription factors and kinase pathways, calcium homeostasis disturbances, inhibition of mitochondrial respiration, and morphological changes (14).
Chemical structure of HNE
HNE is an extraordinarily reactive compound containing three functional groups: a conjugated system of C = C double bond, a C = O carbonyl group which provides a partial positive charge to carbon three and a OH group, the which inductive effect of which is increased at carbon four (fig. 2), (15).
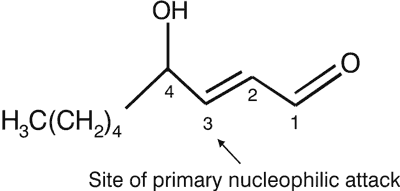
Fig. 2. The chemical structure of 4-hydroxy-2-trans-nonenal (HNE).
Therefore, the nucleophilic attack, e.g., by thiol or amino groups occurs primarily at carbon three and secondarily at the carbonyl carbon one. Furthermore, HNE shows chirality at carbon four, which may also be biologically relevant. Crouzet et al. (16) have shown that (R)- and (S)-HNE are enantioselectively metabolised in rats, and the (S)-enantiomer shows preferential cytotoxicity in normal rat liver cytosol.
Formation of exocyclic adducts to DNA bases via lipid peroxidation and chemical carcinogens
Cyclic etheno adducts were first found to be formed in DNA as a consequence of exposure to environmental carcinogens, such as vinyl chloride and its metabolites, chloroethylene oxide and chloroacetaldehyde, produced after oxidation by cytochrome P450 enzymes (CYP). In addition, anticancer drugs or mucochloric acid, generated from chlorine present in tap water, can also contribute to the formation of etheno bases in DNA. Another possible source of exocyclic adducts is X-ray. X-ray causes fragmentation of carbohydrates, one of the product being phosphoglycoaldehyde, shown to add to deoxyguanosine to form etheno adducts. In tissues of non-exposed rodents and humans several exocyclic adducts have been found and quantified. These include 1,N2-propanodeoxyguanine (PdG) and etheno DNA adducts, such as 1,N6-ethenoadenine (1,N6-εA), 3,N4-ethenocytosine (3,N4-εC), N2,3-ethenoguanine (N2,3-εG) and 1,N2-ethenoguanine (1,N2-εG). It has been postulated that these lesions are formed in mammalian tissues under conditions of lipid peroxidation. The precise mechanism of formation of these lesions is unknown, although formation of etheno adducts, was observed in vitro when deoxyguanosine was exposed to 2,3-epoxy-4-hydroxynonenal (EH). Hydroxynonenal was shown in vitro to bind to deoxyguanosine and form 1,N2-propano-adduct with a hexyl side chain These adducts were found in rodent and human DNA in the range of 1.8-15.8 adducts/108 nucleotides (17).
Ethenoadenine and ethenocytosine have been detected, by immunoaffinity/32P postlabeling, in DNA of untreated rodents and humans at levels ranging from 0.043 to 35 εA per 108 unmodified adenine residues and 0.05 to 24 εC per 108 unmodified cytosines. Using gas chromatography/mass spectrometry (GC/MS) technique Ham and co-workers estimated N2, 3-εdG to range between 50-1720 adducts/106 normal dGuo bases in calf thymus.
Vinyl chloride as an inducer of etheno – DNA adducts
Vinyl chloride -induced mutagenesis depends on oxidative metabolic activation. In mammalian cells, vinyl chloride is activated into chloroethylene oxide by cytochrome P450-dependent microsomal monooxygenases. Chloroethylene oxide binds directly to nitrogen atoms of DNA bases, forming adducts in the following quantitative order: N7-(2-oxoethyl)guanine >> 1,N6-εA > hydroxyethanoguanine > N2,3-εG > 3,N4-εC > 1,N2-εG. Hydroxyethanoguanine undergoes further rearrangement to N2-(2-oxoethyl)guanine. Alternatively, chloroethylene oxide rearranges to form chloroacetaldehyde (CAA). CAA binds to adenine and cytosine in DNA, forming mainly hydroxyethano derivatives, which subsequently dehydrate to 1,N6-εA and 3,N4-εC (fig. 3).
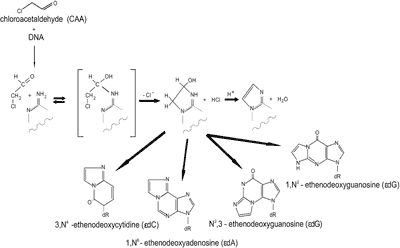
Fig. 3. Schematic presentation of the hydroxyethano derivatives formation induced by vinyl chloride.
In poliribonuclotides, the conversion of hydroxyethanocytosine to εC occurs with a calculated half-life of 15 h, and conversion of hydroxyethanoadenine to εA occurs with a half-life of 1.4 h at 370C at pH 7.25 (18). Formation of hydroxyethano guanosine is also possible, although these derivatives were not detected upon treatment of nucleic acids or nucleosides with CAA. These hydrated forms may also be formed in DNA of animals exposed to vinyl compounds, but their biological significance is poorly elucidated. Reaction of CAA with guanine in DNA favours the formation of N2,3-εG; 1,N2-εG is also formed but with at least 100-fold lower efficiency (18). If N1 is not blocked by hydrogen bonding like in free nucleosides, the CAA induced formation of 1,N2-εG prevails over that of N2,3-εG. The quantitative relationship among etheno adducts induced by CAA in double-stranded DNA was reported to be the following: 3,N4-εC ≥ 1,N6-εA > N2,3-εG >>> 1,N2-εG (18).
A more detailed analysis of the accumulation of ethenobases in relation to the length of exposure was done on rat liver, lung and kidney. εC was found to accumulate in the three organs, whereas εA accumulated in the liver but not in the kidney. In the lung, a steady-state level of εA was attained after 2 weeks of exposure. A more specific, high-resolution GC-MS technique also became available for measuring N2,3-εG. Combining immunoaffinity purification with this technique, Swenberg (19) measured N2,3-εG in liver DNA from adult rats exposed to different doses of vinyl chloride and mice exposed for 1 year to vinyl fluoride. These authors observed dose-related effects on the levels of N2,3-εG formed in hepatic DNA (19) and also measured background levels of N2,3-εG ranging from 6×10-8 to 7×10-7 (molar ratio N2,3-εG /G in a series of 12 DNA samples from human liver). They detected similar levels in unexposed rats. All this places etheno DNA adducts as one of the most important components of the carcinogen/oxidative stress pathway leading to genome instability, cancer and other degenerative processes.
Mutations induced by etheno and propano-DNA adducts
In bacteria and mammals (simian kidney cells), etheno and propano adducts induce base substitutions, frameshift mutations as well as sister chromatid exchanges and chromosomal aberrations. Etheno DNA adducts are moderate inhibitors of DNA synthesis, both in vitro and in bacterial and mammalian cells. However replicative DNA polymerases tend to incorporate non-cognate nucleotides opposite etheno-adducts, which leads to mutations with a frequency, dependent on the source of DNA polymerase. Mutagenic properties of propane adducts have been established for malondialdehyde, acrolein, crotonaldehyde and trans -4-hydroxy-2-nonenal (HNE) (tab. 2).
Table 2. The types of base changes induced by ethenobases and propanobases observed in vitro in E.coli and mammalian cells.
| Lesion | Base changes |
| In vitro | E. coli | Mammalian cells |
| εA | A→G, A→T >A→C | A→G >A→C, A→T | A→G > A→T, A→C |
| β | A→T >A→C | A→G, A→C, A→T | Not Determined |
| εC | C→A, C→T >C→G | C→T, C→A | C→A, C→T >C→G |
| εC•H2O | No incorporation | C→T | Not Determined |
| N2, 3εG | G→A | G→A | G→T, G→A |
| 1,N 2-εG | G→T, G→C | G→T, G→C, G→A | G→A > G→T |
| HO-ethanoG | G→T, G→C | G→T, G→C, G→A | Not Determined |
Malondialdehyde
M1G | G→T, C→T >A→G
M1G→A , M1G→T, M1G→C |
acrolein
α-OH-PdG
γ-OH-PdG | G→T, C→T >A→G |
| crotonaldehyde | C→A, G→T, G→C, C→G, G→A, C→T >A→T>>> T→A |
| Trans-4-hydroxy-2-nonenal (HNE) | G→T, C→A |
DNA repair
To counteract deleterious consequences of DNA damage, the cells developed several repair mechanisms which eliminate from genomes mis-instructive or non-instructive elements, as well as seal DNA breaks. Repair of exocyclic, (ethano and propano DNA adducts is realized by different systems of DNA repair, mainly by base excision repair pathway (Gros et al., 2003), initiated by DNA glycosylases which cleave out the damaged base and initiate the synthesis step and by nucleotide excision repair in mammalian cells in which a larger fragment of damaged DNA strand is removed (12-13 nucleotides in E.coli, 24-32 nucleotides in eucaryota).
Abbreviations: α-OH-PdG: 6R and 6S isomers of 3H-6-hydroxy-3-(β-D-2’-deoxyribofuranosyl)-5, 6, 7, 8-tetrahydropyrido[3, 2-a]purine-9-one, β compound: 4-amino-5-(imidazol-2-yl) imidazole, γ-OH-PdG: 8R and 8S isomers of 3H-8-hydroxy-3-(β-D-2’-deoxyribofuranosyl)-5,6,7,8-tetrahydropyrido[3,2-a]purine-9-one, CV: vinyl chloride, ε-etheno, εA:1,N6-ethenoadenine, εC: 3,N4-ethenocytosine, εG: 1,N2-ethenoguanine, N2,3-εG: N2,3-ethenoguanine, iNOS: inducible nitric oxide synthase, MDA: malondialdehyde, M1-dA: N6 –(3-oxo-propenyl)deoxyadenosine, M1-dC: N4 –(3-oxopropenyl)deoxycitidine, M1-dG: (pirymido[1,2α]purin-10(3H)-one), NOS: nitric oxide synthase, PUFAs: polyunsaturated fatty acids, RNS: reactive nitrogen species.
Piśmiennictwo
1. MacGregor JT, Wehr CM, Hiatt RA et al.: ’Spontaneous’ genetic damage in man: evaluation of interindividual variability, relationship among markers of damage, and influence of nutritional status. Mut. Res 1997; 377: 125-135. 2. Eisen JA, Hanawalt PC: Aphylogenomic study of DNA repair genes, proteins and processes. Mut. Res 1999; 435: 171-213. 3. Oliński R, Gackowski D, Foksiński M et al.: Oxidative DNA damage: assessment of the role in carcinogenesis, artherosclerosis and acquired immunodeficiency syndrome. Free Radic. Biol. Med. 2002; 33: 192-200. 4. Bartsch H, Barbin A, Marion MJ et al.: Formation, detection and role in carcinogenesis of ethenobases in DNA. Drug Metab. Rev 1994; 26: 349-371. 5. Dizdaroglu M, Lava,l J, Boiteux S: Substrate specificity of the Escherichia coli endonuclease III: excision of thymine- and cytosine-derived lesions in DNA produced by radiation-generated free radicals. Biochemistry 1993; 32: 12105-12111. 6. Kochetkov NK, Shibaev VN et al.: New reaction of adenine and cytosine derivatives, potentially useful for nucleic acid modifications. Tetrahedron Lett 1971; 22: 1993-1996. 7. Chaudhary AK, Nokubo M, Reddy GR et al.: Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science 1994; 265: 1580-1582. 8. Nair J, Carmichael PL, Fernando RC et al.: Lipid peroxidation-induced etheno-DNA adducts in the liver of patients with the genetic metal storage disorders Wilson’s disease and primary hemochromatosis. Cancer Epidemiol. Biomarkers Prev. 1998a; 7: 435-440. 9. Horton AA, Fairhurst S: Lipid peroxidation and mechanism of toxicity. Crit. Rev. Toxicol 1987; 18: 27-79. 10. Poli G, Dianzani MU, Cheeseman KH et al.: Separation and characterization of the aldehydic products of lipid peroxidation stimulated by carbon tetrachloride or ADP-iron in isolated rat hepatocytes and rat liver microsomal suspensions. Biochem J 1985; 227(2): 629-638. 11. Benamira M, Singh U, Marnett LJ: Site-specific frameshift mutagenesis by a propanodeoxyguanosine adduct positioned in the (CpG)4 hot-spot of Salmonella typhimurium hisD3052 carried on an M13 vector. J. Biol. Chem. 1992; 267: 22392-22400. 12. Oikawa S, Matsunaga A, Saito T et al.: Apolipoprotein E Sendai (arginine 145-->proline): a new variant associated with lipoprotein glomerulopathy. J Am Soc Nephrol 1997; 8 (5): 820-823. 13. Uchida K, Toyokuni S, Nishikawa K et al.: Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry 1994; 33 (41): 12487-12494. 14. Gadoni E, Olivero A, Miglietta A, Bocca C et al.: Cytoskeletal modifications induced by 4-hydroxynonenal. Cytotechnology 1993; 11 Suppl 1: S62-64. 15. Poli G, Schaur RJ: 4-Hydroxynonenal in the Pathomechanisms of Oxidative Stress. IUBMB Life 2000; 50: 315–321. 16. Crouzet F, Alary J, Rao D et al.: Enantioselective metabolism of (R)- and (S)-4-hydroxynonenal in rats. First International Meeting of the HNE-Club 4-Hydroxynonenal and other Lipid Peroxidation Products. Salzburg, Austria 2002; 3-15 July 2002: 38. 17. Wacker M, Wanek P, Eder E: Detection of 1, N2-propanodeoxyguanosine adducts of trans-4-hydroxy-2-nonenal after gavage of trans-4-hydroxy-2-nonenal or induction of lipid peroxidation with carbon tetrachloride in F344 rats. Chem Biol Interact 2001; 137 (3): 269-283. 18. Kuśmierek JT, Singer B: 1,N2-ethenodeoxyguanosine: properties and formation in chloroacetaldehyde-treated polynucleotides and DNA. Chem Res Toxicol 1992; (5):634-638. 19. Swenberg JA, Bogdanffy MS, Ham A et al.: Formation and repair of DNA adducts in vinyl chloride- and vinyl fluoride-induced carcinogenesis. IARC Sci Publ. 1999; 150: 29-43.


