*Labib Zair1, Jędrzej Skrobot2, Mariola Marchlewicz3, Jarosław Lichota1, Leonard Gugała4, Magdalena Kuczyńska5, Samir Zeair6, Ewa Duchnik7, Karol Tejchman1, Barbara Wiszniewska8, Jerzy Sieńko1, Tadeusz Sulikowski1, Marek Ostrowski1, Mirosława El Fray2
Abdominal hernia repair surgery with the new injectable polymer biomaterials
1Clinic of General and Transplantation Surgery, Pomeranian Medical University, Szczecin
Head of Clinic: prof. Marek Ostrowski, MD, PhD
2Division of Biomaterials and Microbiological Technologies, West Pomeranian University of Technology, Szczecin
Head of Division: Mirosława El Fray
3Department of Aesthetic Dermatology, Pomeranian Medical University, Szczecin
Head of Department: prof. Mariola Marchlewicz, MD, PhD
4Veterinary Clinic, Szczecin
Head of Clinic: Leonard Gugała
5Independent Longterm Care Unit, Pomeranian Medical University, Szczecin
Head of Unit: Bożena Mroczek, MD, PhD
6Clinic of General, Vascular and Transplantation Surgery, Maria Skłodowska-Curie Hospital, Szczecin
Head of Clinic: Samir Zaeir
7Department of Dermatology and Venereology, Pomeranian Medical Univeristy, Szczecin
Head of Department: prof. Romuald Maleszka, MD, PhD
8Department of Histology and Embryology, Pomeranian Medical Univeristy, Szczecin
Head of Department: prof. Barbara Wiszniewska, MD, PhD
Summary
Introduction. The new injectable polymer biomaterials offer a completely innovative approach to hernia repair surgery. This study evaluated the potential application of injectable polymer biomaterials in hernia treatment using an animal model. Local connective tissue reactions associated with the new injectable polymer biomaterials were compared with the reactions provoked by commercial polypropylene mesh.
Aim. Evaluation of the potential application of injectable polymer biomaterials in the treatment of abdominal wall hernias using an animal model.
Material and methods. Five groups of rabbits with previously-created hernias were treated with new biomaterials injected in nonpolymerized form into hernial openings. The same animals had previously polymerized discs surgically implanted in the dorsal subcutaneous area. The rabbits were kept under clinical observation for 28 days, then sacrificed under anesthesia. Samples of subcutaneous tissue were taken from the hernia region and from the vicinity of the polymerized biomaterial discs implanted in the dorsal area for histological examination.
Results. In groups 1, 2 and 3, hernia treatment results, laboratory tests and histological examination of the tissues previously taken from the operated hernia sites were similar to the results obtained in the control group. In groups 1, 2 and 3, the numbers of fibrocytes and neutrophils in the vicinity of the subcutaneously-implanted discs in the dorsal area were similar to those in the hernial region treated with mesh in the control group.
Conclusions. Biomaterials 188-UR/PEG-DA 85/15, P1838-DMA and 1838_UR can be used in animals in the near future as possible abdominal wall hernia treatments. These biomaterials were as efficient as the polypropylene mesh used in the control group for treating abdominal wall hernias.
INTRODUCTION
Hernia repair represents a considerable proportion of the operations performed in general surgery. The number of specific operations reflects hernia incidence in the general population; typically, approximately 80% of repairs are inguinal, 10% incisional, 5% femoral, 4% umbilical, and < 1% represent other types of repairs. Surgical efforts are directed towards increasing efficacy, which, particularly in hernia surgery, converts into safety, lowering complication rates, shortening the length of the hospital stay, and lowering the recurrence rate. These goals are achieved by improvements in surgical procedures, the materials used, and general perioperative care (i.e., minimally invasive surgery with laparoscopy as its major part, tension-free procedures, adequate prophylaxis and the use of certain artificial materials in recreating abdominal wall integrity).
Our interest is directed on biomaterials. Currently, the most common is polypropylene mesh, and Lichtenstein’s inguinal hernia repair remains a gold standard technique. However, in our study, we decided to examine the use of liquid, photoinduced stiffening polymers, such as 1838-DMA, P1838-UR, PDEGA-UR and PEG-DA, in an experimental model of rabbits with artificially induced hernias.
AIM
1. To evaluate the potential application of injectable polymer biomaterials in the treatment of abdominal wall hernias using an animal model.
2. To compare tissue reactions elicited by the new polymer biomaterials and a commercial polypropylene mesh.
3. To determine the influence of the new injectable biomaterials on the kidneys and liver.
MATERIAL AND METHODS
The study group consisted of 20 New Zealand rabbits; there were 10 males and 10 females, with an average weight of 2.5 ± 0.6 kg. During stage 1 (tab. 1), we surgically created abdominal wall hernias in the group of rabbits. First, we established access to the jugular or femoral vein for blood sample collection. General anesthesia was induced by the intravenous administration of Domitor (0.2 ml IV) and ketamine (20 mg [0.2 ml] IV; Orion Pharma). After shaving the fur and disinfecting the skin, a ventral midline incision was made below the navel; the 5-cm incision went through the skin, subcutaneous tissue, fascia and muscle flap, uncovering the peritoneum. Afterward, the skin was closed by primary, continuous suture. The rabbits were observed for 30 days, allowing wound healing and hernias to occur.
Table 1. Study schedule.
| | Procedures | Time |
| Stage I | General anaesthesia
Laboratory examination
Weight prior to surgery
Creation of hernia | 30 days between first and second surgery
Everyday clinical observation
Motor activity
Wound healing
Nutrition |
| Stage II | General anaesthesia
Laboratory examination
Weight prior to surgery
Hernia repair surgery | 28 days between second operation and autopsy
Everyday clinical observation
Motor activity
Wound healing
Nutrition |
| Stage III | General anaesthesia
Laboratory examination
Weight prior to dissection
Autopsy
Histological examination | Clinical observation of the location of treated hernia from exterior and from the side of abdominal cavity |
During stage 2 (tab. 1), the rabbits were randomized into five groups of four rabbits each for treatment with the following polymers: group 1 – P1838-UR/PEG-DA 85/15; group 2 – P1838-DMA; group 3 – P1838-UR, 4; group 4 – PDEGA-UR, and group 5 – control group, standard polypropylene mesh. Hernia repair surgery was performed under general anesthesia as described above. Blood samples for laboratory examination were collected from the jugular or femoral vein. The surgical field was shaved and disinfected, and the skin and subcutaneous tissue were incised over the hernial sac (fig. 1-3). The dissected sac was discharged into the abdominal cavity, and the appropriate polymer biomaterial was introduced into the hernial defect. The amount of biomaterial used per application varied from 1-2 cm3. Groups 1-4 received 5 minutes of irradiation with a UV lamp, which turned the liquid into an elastic, porous sponge (fig. 4 and 5). The subcutaneous and cutaneous tissues were closed using a continuous suture. In group 5, the mesh was not exposed to UV irradiation, and it was not fixed with sutures. After hernia repair, the rabbits were kept under clinical observation for 28 days.
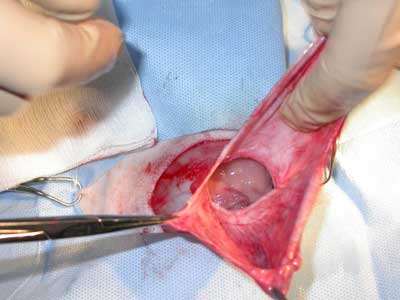
Fig. 1. Hernia sack opened.
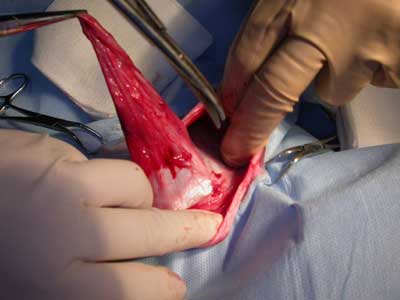
Fig. 2. Hernia sack during preparation.
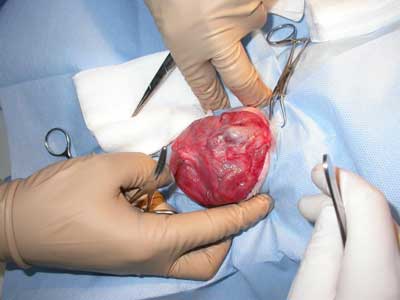
Fig. 3. Hernia sack with contents.
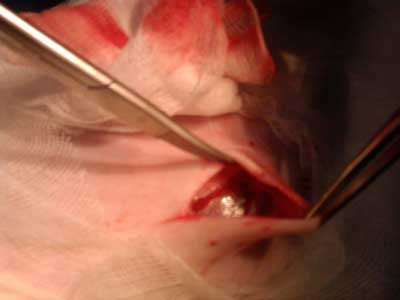
Fig. 4. Hernia with biomaterial inserted.
Fig. 5. Groups 1-4 received 5 minutes of irradiation with a UV lamp, which turned the liquid into an elastic, porous sponge.
During stage 3 (tab. 1), the rabbits were sacrificed using 3 ml (480 mg) of Morbital while under anesthesia (medetomidine plus ketamine; Orion Pharma). Postmortem samples were collected from the tissues surrounding the hernia repair site and from the kidneys and the liver.
The biomaterials used in the current study were synthetized from fatty acids present in plant oils. Depending on the type and variety of the material, up to 80% of its mass was derived from renewable sources. The materials 1838-DMA and P1838-UR contain a fatty acid core. PDEGA-UR is a derivative of poly[di(ethylene glycol) adipate], and PEG-DA is diacrylated poly(ethylene glycol) (fig. 6). All of the materials had (meth)acrylic end-groups, making them susceptible to polymerization with UV light. The compositions used for hernia repair also contained the photoinitiator Irgacure 819 (Ciba). The polymers we-re synthesized under mild conditions. The process was carried out in an organic solvent under normal pressure and in the temperature range of 0 to 40°C (1). These biomaterials were created specifically for this project and have not yet been tested in other applications.
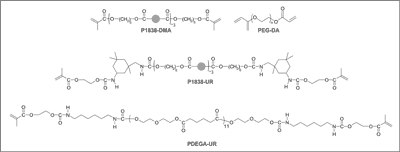
Fig. 6. Biochemical structure.
All rabbits were under the constant supervision of a veterinary surgeon. During the study, the following were recorded: vital signs, weight, food consumption, motor activity, the presence of swelling and redness around the wound, and the presence of fluid under the scar. Wound control included observation of both the external side and the peritoneal side of the treated hernia for assessment of hernia relapse. Blood was collected at every stage to measure aspartate transaminase (AST), gamma-glutamyl transferase (GGTP) activity and creatinine concentration.
Samples for histologic evaluation were preserved in freshly prepared 4% paraformaldehyde and then embedded in paraffin. For the purposes of morphological analysis, a slide series was made at 3-5 μm intervals, and the tissues were stained using hematoxylin and eosin. The cross-sections were dyed with the intent of calculating the numbers of various types of cells (fibrocytes, neutrophils, eosinophils, lymphocytes, macrophages). A 10 × eyepiece with a square visor was used for examination. For each animal’s tissue samples, the number of cells was calculated from 50 randomly selected fields; this evaluation was performed using 40 × augmentation and a Zeiss brand microscope. The surface area of each square was 1225 μm2 in size (35 × 35 mm) within the framework of the target. All morphometric measurements were obtained using AxioVision Relative software, version 4.6 (Zeiss, Axioscop, Germany).
Statistical analysis was performed with the Statistica 10 software package; the Shapiro-Wilk and Mann-Whitney tests were used for comparisons, with the level of significance set at p < 0.05. The study was conducted after obtaining the consent of the bioethics committee.
RESULTS
This study provided data drawn from: (1) clinical observation of the rabbits; (2) biochemistry tests; (3) histopathologic evaluation of the kidneys and liver; and (4) histopathologic evaluation of the tissues surrounding the hernia. There were no differences between the groups in motor activity, daily food intake or average weight gain. Would healing was undisturbed in groups 1, 2, 3 and 5, while wound inflammation was observed in group 4. Two deaths, caused by pneumonia and hernia incarceration, were observed during stage 1.
Biochemical tests
No statistically significant differences in AST, GTTP and creatinine concentration were found between the rabbits from groups 1, 2, 3 and 4 and the rabbits from the control group (group 5) during the three stages of the study.
Morphology of the examined tissues
Liver and kidney morphology
The liver and kidneys from the rabbits treated with polypropylene mesh (the control group) (fig. 7A, 7C) and from the rabbits treated with the new polymer biomaterials (groups 1, 2, 3, 4) presented typical morphology (fig. 7B, 7D). In the liver, the hepatocytes were grouped in interconnected plates to form liver lobules with portal spaces in the periphery and a central vein in the center. Plates of hepatocytes were directed from the periphery to the center of the lobule. Liver sinusoids were visible in the space between the plates. In all of the livers, small numbers of leucocytes were visible in the connective tissue surrounding the blood vessels and bile ducts in the portal spaces. No leucocytes were found near central veins.
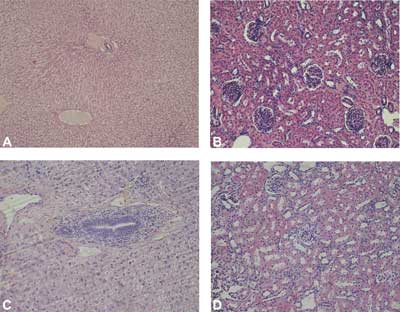
Fig. 7. The morphology of liver (A) and kidney (C) in rabbits of control group (group 5), and liver (B) and kidney (D) of rabbits treated with new injectable material (group 1). H-E, objective magnification x 20.
The kidneys were composed of cortex and medulla. Nephron-related elements were present in the cortex: renal corpuscles, proximal convoluted tubules and distal convoluted tubules. Medullary rays penetrating the cortex were visible. Collecting tubules, loops of Henle and capillaries were observed in transverse sections of the medulla. There was no inflammatory cell infiltration in the interstitium of the renal cortex and medulla.
Morphology of connective tissue around the treated hernia
Small numbers of leucocytes were visible in the connective tissue surrounding the treated hernias in rabbits from group 5 (the control group) (fig. 8A) and in rabbits from groups 1 (fig. 8B) and 2 (fig. 8C). In groups 3 and 4, massive inflammation was observed in the connective tissue near the site where the biomaterials had been injected compared with the connective tissue of rabbits from the control group (group 5) (fig. 8D).
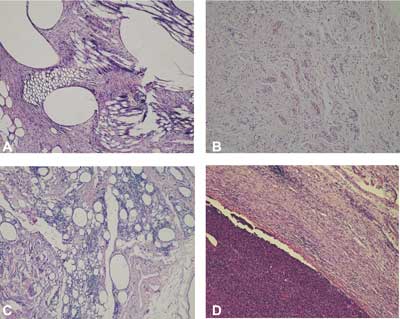
Fig. 8. The connective tissue around implanted material in rabbits treated with polypropylene mesh (A), and with new injectable material in rabbits of group 1 (B), group 2 (C), group 3 (D). Visible elements of connective tissue – fibroblasts, collagen fibers, blood vessels surrounding polypropylene mesh (M)(A); leukocytes in tissue surrounding new injectable material in rabbits of group 2 (C), and massive inflammatory infiltration around the injected material in rabbits of group 3 (D). H-E, objective magnification x 10.
Morphometric analysis
Table 2 presents the numbers of various cell types in the connective tissue surrounding the treated hernias in rabbits from group 5 (the control group) and from groups 1, 2, 3 and 4.
Table 2. Numbers of fibrocytes, neutrophils, lymphocytes, macrophages and eosinophils in the connective tissue around the surgically treated hernias.
| ↓ Group | Cells → | Fibrocytes | Neutrophils | Lymphocytes | Macrophages | Eosinophils |
Group I
| M | 54.23 | 0 | 24.39 | 1.22 | 1.45 |
| Q1–Q3 | 34.75–70.95 | 0.00–0.00 | 23.56–28.22 | 0.87–1.26 | 1.34–2.00 |
| X ± SD | 52.27 ± 19.80 | 0.00 ± 0.00 | 26.23 ± 5.38 | 1.07 ± 0.28 | 1.65 ± 0.57 |
Group II
| M | 61.34 | 0 | 28.57 | 0 | 10.09 |
| Q1–Q3 | 24.86–79.31 | 0.00–0.00 | 20.24–50.28 | 0.00–0.56 | 4.60–24.30 |
| X ± SD | 54.03 ± 27.64 | 0.00 ± 0.00 | 33.09 ± 16.32 | 0.22 ± 0.31
Gr1vs gr2** | 12.66 ± 11.21 |
Group III
| M | 45.45 | 0.78 | 33.57 | 0 | 20.98 |
| Q1–Q3 | 45.45–46.5 | 0.00–0.81 | 33.57–34.89 | 0.00–0.00 | 17.83–20.98 |
| X ± SD | 45.28 ± 2.06 | 0.71 ± 0.80 | 33.96 ± 3.67 | 0.33 ± 0.73 | 19.72 ± 5.83
Gr1vs gr3** |
Group IV
| M | 44.44 | 2.74 | 42.55 | 0.71 | 12.06 |
| Q1–Q3 | 43.26–47.95 | 1.42–3.25 | 35.65–43.09 | 0.68–0.87 | 4.79–18.30 |
| X ± SD | 44.97 ± 10.42 | 2.31 ± 1.10
Gr1vs gr4**
Gr2 vs gr4**
Gr3 vs gr4* | 39.82 ± 4.63
Gr1vs gr4*
| 0.78 ± 0.58 | 12.12 ± 8.11
Gr1vs gr4** |
Group V
| M | 68.4 | 0 | 29.3 | 1.03 | 1.02 |
| Q1–Q3 | 60.82–68.4 | 0.00–2.06 | 29.3–34.02 | 0.85–2.25 | 0.00–2.06 |
| X ± SD | 64.2 ± 7.26
Gr4 vs gr5* | 1.61 ± 2.60 | 31.76 ± 3.92 | 1.28 ± 0.97 | 1.13 ± 1.17
Gr4 vs gr5** |
M – median; Q1–Q3 – lower–upper quartile; X – arithmetic mean; SD – standard deviation. Asterisks indicate statistically relevant differences per the Mann-Whitney U-test.
*p < 0.05; **p < 0.01
1. The average number of fibrocytes was the highest in group 5 and the lowest in group 4; this difference was statistically significant.
2. The average number of neutrophils was the highest in group 4 and the lowest in groups 1 and 2; there was a statistically significant difference between group 4 and groups 1, 2 and 3.
3. The average number of lymphocytes was the highest in group 4 and the lowest in group 1; this difference was statistically significant.
4. The average number of macrophages was the highest in group 5 and the lowest in group 2; there was a statistically significant difference between group 1 and group 2.
5. The average number of eosinophils was the highest in group 3 and the lowest in group 5; statistically significant differences were found between groups 1 and 3, 1 and 4, and 4 and 5.
DISCUSSION
Hernias occur in 27% of men and 3% of women (1). For many years, repair surgery has utilized biomaterials in the form of polypropylene or polyester meshes. Since 1978, when Lichtenstein introduced polypropylene mesh in hernia treatment (2, 3), a significant improvement has been observed in both the quality of the meshes applied (polyester and polytetrafluoroethylene meshes that are resistant to infections) and the surgical techniques used (tension and tension-free laparoscopic repairs) (4, 5). The use of biomaterials in hernia treatment has reduced the number of relapses to less than 1% (6, 7). The benefits resulting from laparoscopic methods that employ a mesh and from open, tension-free methods have been demonstrated by Swedish authors (8). Relapses after laparoscopic surgeries occur in 2% of patients compared with 5% of patients who undergo surgery utilizing the Shouldice technique. In comparison with tension methods, postoperative progress is more favorable in patients who undergo surgery employing the Lichtenstein technique (9-11): these patients experience a shorter hospitalization period (12), less postoperative pain, less frequent use of analgesic drugs, and a faster recovery (9, 11, 12). Presently, most hernia repair surgery is performed with a tension-free technique employing a mesh and using inguinal access (13). In order to fix the mesh, nonabsorbable surgical sutures and clips are applied, as well as fibrin glue, which was used in laparoscopic surgery by Kathouda (14).
The biomaterials that have been recently developed are resistant to enzymatic and hydrolytic degradation under simulated biological conditions. This resistance may be easily increased or decreased depending on the material’s chemical composition. For example, if an easily degradable filler is introduced into the matrix, this will result in the creation of a porous structure. In such an instance the material will have the consistency of honey and may be delivered to the application site through a small-diameter canal; it may also be given the shape and form that is desired. The cross-linking of this material occurs through low intensity UV irradiation. This type of material has been tested and found to be safe for the organism (15, 16).
In vitro studies performed in a separate project of ours revealed that the new biomaterials have no toxic influence on cultured fibroblasts. Clinical observations in the current study revealed no statistically significant differences in weight gain, nutrition, motor activity or wound healing between groups 1, 2, 3 and 5. There were also no hernia relapses, although the postoperative observation period was too short to evaluate the significance of this finding. These results were not surprising because the biomaterials used in these groups had a similar chemical structure; however, the polymers’ viscosity differed, potentially affecting clinical observations. Significant differences in comparison with group 4 were also expected. The biomaterial used in this group was completely synthetic and was recognized by the immunological system as a foreign antigen, which resulted in a significant inflammatory response. Group 4, in contrast to the other groups, showed inflammation, intra-abdominal infection, wound dehiscence and hernia relapse, although motor activity, body weight gain and food consumption remained unchanged. These observations may be explained by the ability of the peritoneum to limit the inflammation. Histopathologic evaluation of the kidneys and liver revealed no significant differences compared with the other groups.
Because of similar results in groups 1, 2, 3 and 5 in terms of clinical observations, laboratory tests and histologic evaluation, we came to the conclusion that the newly developed polymers are characterized by safety comparable to that of standard polypropylene mesh. Nonsignificant differences in inflammatory cell distribution were observed. This study did not allow for the evaluation of overall efficacy because of small sample sizes and a lack of long-term follow-up. Nevertheless, the method that we used enables simple and easy management of a hernial defect because the whole treatment process, from the minimally invasive introduction of the material at the hernial site to the material’s transformation from liquid to elastic form, is fast and readily controlled (16). Certainly, there is a difference between the procedures used with polypropylene mesh versus liquid biomaterials. Mesh requires proper implementation and fixation, which, depending on the conditions, may be difficult, especially when using laparoscopy. Moreover, mesh remains unchanged in the body, sometimes leading to inflammatory complications or pressure on anatomic structures (e.g., spermatic cord, nerves, vessels), which may result in chronic pain or difficulties in performing reoperations. Biomaterials have the advantage of allowing for relatively more comfortable implementation because they do not require fixation; their final form is achieved by UV radiation, which provides time for shaping; and they undergo enzymatic biodegradation in vivo. The spongy structure of UV-linked biomaterials fills the space both under and over the fascia, adjusting to the surrounding space without compression; the pores create an environment for new connective tissue cells. With time, biomaterials are fully replaced by connective tissue, which is intended to prevent hernia relapse in the same way that connective tissue grown on polypropylene mesh does. These advantages relative to safety, minimally invasive surgery and satisfactory long-term results may make hernia repairs utilizing these new biomaterials competitive with standard procedures. However, the efficacy achieved with these materials requires further research.
CONCLUSIONS
1. The use of biomaterials P1838-UR/PEG-DA 85/15 (group 1), P1838-DMA (group 2) and P1838-UR (group 3), when applied according to our study protocol, appears to be successful in treating abdominal hernias in rabbits.
2. The local tissue reaction caused by these three biomaterials is similar to that caused by polypropylene mesh (group 5) in rabbits.
3. These three biomaterials do not affect the kidneys or liver of rabbits based on histopathologic examination, creatinine concentration, and GGTP and AST serum activity after 30-day observation; the results of these examinations were similar to those found with implemented polypropylene mesh (group 5).
Our research was supported by the Ministry of Science and Higher Education, grant nr N507 434734.
Piśmiennictwo
1. El Fray M, Skrobot J, Bolikal D, Kohn J: Synthesis and characterization of telechelic macromers containing fatty acid derivatives. React & Funct Polym 2012; 72(9): 781. 2. Primatesta P, Goldacre MJ: Inguinal hernia repair. Incidence of elective and emergency surgery, readmission and mortality. Int J Epidemiol 1996; 25: 835-839. 3. Witkowski P: Surgical treatment of inguinal hernia. Bassini and Rudkow methods, prospective randomized study. Akademia Medyczna, Gdańsk 1999. 4. Haapaniemi S, Gunnarsson U, Nordin P et al.: Reoperation after recurrent groin hernia repair. Ann Surg 2001; 234: 122-126. 5. Chróścicki S, Falkowski W: Results of hernia treatment with biomaterials. Pol Przeg Chir 1969; 10: 1356-1358. 6. Hair A, Peterson C, Wright D et al.: What effects does the duration of an inguinal hernia have on patient symptoms? Jam Coll Surg 2001; 193: 125-129. 7. Bay-Nielsen M, Nordin P, Nillson E et al.: Operative findings in recurrent henia after a Lichtenstein procedure. Am J Surg 2001; 182: 134-136. 8. Maggiore D, Muller G, Hafanaki J: Bassini vs Lichtenstein: two basic techniques for inguinal hernia treatment. Hernia 2001; 5: 21-24. 9. Fleming WR, Elliott TB, Jones RM et al.: Randomized clinical trial comparing totally extraperitoneal inguinal hernia repair with the Shouldice technique. Br J Surg 2001; 88: 1183-1188. 10. Lichtenstein IL, Schulman AG, Amid PK, Montllor MM: Cause and prevention of postherniorrhaphy neuralgia: protocol for treatment. Am J Surg 1988; 155: 786-790. 11. Rychlewski D, Wojczys R: Pain after inguinal hernia repair. Chirurgia Polska 2007; 9(3): 180-185. 12. Luijendijk RW, Hop WC, van den Tol MP et al.: A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 2000; 343: 392-398. 13. Pasieka Z: Comparison of early and long-term results of inguinal hernia repairs with selected surgical techniques. Chirurgia Polska 2004; 6(1): 19-25. 14. Bobrzyński A, Budziński A, Rembiasz K: Laparoscopy in abdominals hernias treatment. Przegląd lek 2001; 58: 45-50. 15. Katkhouda N, Mavor E, Friedlaner MH et al.: Use of fibrin sealant for prosthetic mesh fixation in laparoscopic extraperitoneal inguinal hernia repair. Ann Surg 2001; 233: 18-25. 16. El Fray M, Zair L, Skrobot J: Application of composition containing telechelic macromer and photoinitiator for producing implant for hernia repair. Pending U.S. Patent Application No. 13/727,876 (2012).







