*Terèzia Seres1 , Zoltán Szakács2, Nóra Pető2, Éva Kellős1, Veronika Fáy4, Jelena Karaszova4, Andrea Kontra4, Olivia Lalátka4, Gyula Domján3
Sleep-related breathing disorders in Hungarian patients with Parkinson’s disease
1Doctoral School, Semmelweis University, Budapest, Hungary
Head of Department: Gyula Domján, MD
2Department of Neurology, Hungarian Defence Forces Military Hospital, Budapest, Hungary
Head of Department: Zoltán Szakács, MD, PhD
3Institute of Health Care Development and Clinical Methodology, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
Head of Institute: Professor Gyula Domján, MD, PhD
4Rehabilitation Centre, Combined Szent István and Szent László Hospital, Budapest, Hungary
Head of Centre: Veronika Fáy, MD
5Department of Nursing, Faculty of Health Sciences, Semmelweis University, Budapest, Hungary
Head of Department: Zoltán Balogh, MD
Summary
Introduction. Typical symptoms of Parkinson’s disease (PD) are motor symptoms. However, the non-motor symptoms, which may occur in any phase of the disease, are now in the center of the clinical attention. These symptoms include neuropsychiatric dysfunctions, dysautonomy, sleep disorders, and sensory symptoms, such as pain. Sleep disorders are common in PD patients.
Aim. The aim of our study was to estimate the prevalence and characteristics of obstructive sleep apnea syndrome (OSAS) in patients with PD. We also wanted to analyze the sleep architecture in Parkinson’s disease using polysomnography.
Material and methods. 50 patients who had visited the Neurology Department of Hungarian Defence Forces Military Hospital between February 2014 and April 2016 were recruited for the study. Every patient with idiopathic Parkinson’s disease stage 1 to 3 was included, regardless of their sleeping complaints. Every patient underwent nocturnal, in-laboratory polysomnography, the results of which were subsequently assessed by a somnologist. Sleep stages were distinguished and the Apnea-Hypopnea Index (AHI) was calculated according to the recommendations of the Task Force of the American Academy of Sleep Medicine.
Results. The total in-laboratory sleep time ranged from 189 minutes to 501 minutes, with the mean value of 298 minutes. Total sleep time was reduced (< 5 hours) in 28 patients (56%). Sleep latency was prolonged (< 0.5 hours) in 33 patients (> 66%). In older patients (≥ 75 years old), the sleep latency was longer. The normal sleep efficiency of > 85% was seen in only 8 patients. The sleep efficiency ranged from 56% to 89%, with a mean value of 74.1%. 9 patients in our study group had 3 rapid eye movement (REM) sleep episodes, 37 patients had 2 REM episodes and 4 patients had only 1 REM episode. There was a negative correlation between the score on Epworth Sleepiness Scale (ESS) and the number of REM episodes. REM sleep onset latency was prolonged (> 2 hours) in 82% (n = 42) of our patients. Periodic limb movements in sleep (PLMS) were seen in 18 patients. There was a negative correlation between age and PLMS index. All the patients in our study who had been diagnosed with restless leg syndrome (RLS) had PLMS . Sleep latency was prolonged in 7 out of 17 patients suffering from RLS. 64% (n = 32) of our patients were diagnosed with OSAS. The prevalence of severe, moderate and mild OSAS was 22%, 32% and 10%, respectively. Patients with moderate and severe OSAS (AHI > 15 hours) had higher age than patients without OSAS (p < 0.005). The mean ESS score was higher in OSAS patients (p = 0.05). Snoring was present in 78% of the OSAS patients. Apnea witnessed by a partner was the most specific symptom, present in 92% of OSAS patients. We did not find significant differences between the groups with and without OSAS in regard of UPDRS (unified PD rating scale) and Hoehn & Yahr’s modified evaluation scale and Schwab & England’s functional evaluation scale.
Conclusions. OSAS is a common sleep disorder in PD patients. It has a higher prevalence in older PD patients and it is associated with greater daytime sleepiness. Snoring is the most sensitive symptom, and apneas witnessed by a partner are the most specific symptom of OSAS in PD patients.
Introduction
Typical symptoms for Parkinson’s disease (PD) are motor symptoms. However, the non-motor symptoms, which may occur in any phase of the disease, are becoming in the center of the clinical attention. These symptoms include neuropsychiatric dysfunctions, dysautonomy, sleep disorders, and sensory symptoms, such as pain (1). Disorders of sleep and wakefulness occur in about 60-98% of patients with PD. The majority of research on the prevalence and nature of these disorders was performed in patients treated with antiparkinsonian drugs, and, therefore, the side effects of the therapy were interfering with the true picture of sleep disorders (2). The spectrum of sleep disorders in PD patients is broad and includes insomnia, parasomnia and hypersomnia.
The main symptoms of insomnia are difficulty in maintaining sleep, associated with nocturia, rapid eye movement (REM) sleep behavior disorder (RBD), night cramps, akinesia and tremor. The prevalence of hypersomnia in PD patients not receiving antiparkinsonian drugs was comparable to the prevalence of hypersomnia in general population. In some studies, hypersomnia is considered independent from PD, not associated with the quality of night’s sleep or concomitant therapy. Parasomnias in PD patients are manifested primarily by REM-sleep behavior disorder (RBD) in the early stage of the disease, and it has been proven to be a predicting factor for the development of PD in asymptomatic patients.
However, there is no conclusive evidence to support the relationship between PD and the prevalence of obstructive sleep apnea (OSA) (3). Nevertheless, the prevalence of Obstructive Sleep Apnea Syndrome (OSAS) among patients with PD ranges from 20% to 66%. OSAS in PD patients have different characteristics than in general population (4).
Aim
The aim of our study was to estimate the prevalence and characteristics of obstructive sleep apnea syndrome (OSAS) in patients with PD. We also wanted to analyze the sleep architecture in Parkinson’s disease using polysomnography.
Material and methods
50 patients (29 men and 21 women; mean age 71.9 ± 11.8 years; mean Hoehn-Yahr stadium 1.9 ± 0.8) who had visited the Neurology Department of The Hungarian Defence Forces Military Hospital between February 2014 and April 2016 were recruited for the study. Every patient with idiopathic Parkinson’s disease stage 1 to 3 was included, regardless of their sleeping complaints. Every patient suffered from bradykinesia and at least one another typical symptom of PD: resting tremor, rigidity, and impairment of the postural reflexes. Patients with atypical symptoms, including pyramidal signs, cerebellar symptoms, dyspraxia, and autonomic dysfunction, were excluded from the study. Patients with depression and dementia diagnosed according to DSM-IV criteria were also excluded. None of the patients nor their relatives reported visual hallucinations or fluctuating cognition, which was of importance, as these symptoms are the typical signs of Dementia with Lewy Bodies (4). The medical history of none of the patients revealed exposition to toxic materials, head injury, encephalitis or cerebrovascular disease. 16 healthy controls with matching age and gender (10 men and 6 women; age 62.31 ± 6.87 years) who did not suffer from sleep disorders were included (10 men and 6 women; average age SD, 62.31 ± 6.87 years).
44 patients were treated with a dopaminergic medicine: 39 with levodopa (average daily dosage: 510 mg); 13 with selegiline (average daily dosage: 5 mg); 12 with pramipexole (average daily dosage: 4.3 mg); 3 with bromocriptine (average daily dosage: 18 mg); 2 with amantadine (average daily dosage: 150 mg); 2 with ropinirole (average daily dosage: 14 mg). One patient had withdrawn medications three months before the study and 5 patients had never been treated for Parkinson’s disease.
No person from the study group nor the control group received benzodiazepines, tricyclic antidepressants or SSRIs. Furthermore, no person from the control group used any medication that could have influenced the sleeping pattern.
The participants underwent a consultation with a somnologist, who administered structured interviews assessing sleep disorders and PD, using the Epworth‘s Daytime Sleepiness Scale (ESS), the unified PD rating scale (UPDRS) and the Hoehn & Yahr’s modified evaluation scale. Each subject was then referred to an in-laboratory polysomnography (PSG), which is the gold standard for the diagnosis of obstructive sleep apnea/hypopnoea syndrome (OSAHS) (5).
Every patient underwent nocturnal, in-laboratory polysomnography. The lights were extinguished at the regular time of getting into bed of the patient (between 21:42 and 23:29). During the recording, data from the following diversions was collected to define sleeping stages: right and left side electrooculogram (EOG), submental EMG and two-two centralis (C3-A2, C4-A2) as well as occipitalis (O1-A2, O2-A1) EEG diversions. When RBD is suspected in a patient, additional monitoring during polysomnography must be ensured. Apart from the obligatory video monitoring, EMG channels recording the tone on all the four limbs (musculi tibialis anterior, soleus and biceps brachii bilaterally) and in the chin muscle are recommended. We also used an infrared video recorder to detect patients’ movements during REM sleep. To define REM sleep stage, we used exclusively EEG and electrooculogram. REM latency, defined as the interval from the sleep onset to the first appearance of REM sleep, was also measured. The end of the REM sleep period was indicated on the grounds of specific EEG graphoelements indicating other sleep stages (K-complexes, sleep spindles or arousal spikes), as well as on the grounds of continuous lack of rapid eye movements longer than three minutes. We repeated the procedure in the control group. To define sleep stages from 1 to 4, epochs of 30 seconds width were used and modified Rechtschaffen and Kales scoring criteria were applied.
The results were assessed by a sleep specialist. Sleep stages were distinguished and the Apnea-Hypopnea Index (AHI) was calculated according to the recommendations of the Task Force of the American Academy of Sleep Medicine (6).
Results
The total in-lab sleep time ranged from 189 minutes to 501 minutes, with the mean value of 298 minutes. Total sleep time was reduced (< 5 hours) in 28 patients (56%) (tab. 1). Sleep latency, i.e. the time from going to bed to falling asleep, was prolonged (< 0.5 hours) in 33 patients (> 66%). Normal sleep latency is 15-20 minutes (7). Sleep latency range in our study was 8-128 minutes, and mean sleep latency was 30.3 minutes. An increase in this parameter suggests a pathology of the initiation of sleep.
Tab. 1. Sleep characteristics of the entire study population
| Parameter | N | Minimum | Maximum | Mean | Std. Deviation |
| Age | 50 | 48 | 91 | 72.00 | 10.30 |
| ESS | 50 | 0 | 21 | 10.10 | 6.12 |
| Total Sleep Time | 50 | 189 | 501 | 298.28 | 57.58 |
| Sleep latency | 50 | 8 | 128 | 30.28 | 19.40 |
| Sleep efficiency | 50 | 56 | 89 | 74.04 | 8.59 |
| REM episodes | 50 | 1 | 3 | 2.10 | 0.51 |
| REM sleep onset latency | 50 | 87 | 258 | 144.08 | 31.86 |
| PLMS index | 50 | 0 | 18 | 3.78 | 4.81 |
In older patients (≥ 75 years old), the sleep latency was longer (tab. 2). Sleep efficiency is quotient of the time spent sleeping by time spent in bed. The normal sleep efficiency of > 85% was seen in only 8 patients (8). The sleep efficiency ranged from 56% to 89%, with a mean value of 74.1%.
Tab. 2. Sleep characteristics by age
| Parameter | Age
0: < 75 years
1: ≥ 75 years | N | Mean | Std. Deviation | Std. Error Mean |
| Total Sleep Time | 0 | 27 | 298.96 | 62.66 | 12.059 |
| | 1 | 23 | 297.48 | 52.37 | 10.918 |
| Sleep latency* | 0 | 27 | 27.93 | 22.43 | 4.317 |
| 1 | 23 | 33.04 | 15.14 | 3.158 |
| Sleep efficiency | 0 | 27 | 74.89 | 8.26 | 1.589 |
| | 1 | 23 | 73.04 | 9.05 | 1.887 |
| REM episodes | 0 | 27 | 2.11 | 0.51 | 0.097 |
| | 1 | 23 | 2.09 | 0.51 | 0.107 |
| REM sleep onset latency | 0 | 27 | 147.56 | 38.85 | 7.477 |
| | 1 | 23 | 140.00 | 21.07 | 4.394 |
| PLMS index* | 0 | 27 | 5.33 | 5.38 | 1.035 |
| 1 | 23 | 1.96 | 3.30 | 0.687 |
*statistically significant differences (p < 0.05)
9 patients in our study group had 3 rapid eye movement (REM) sleep episodes, 37 patients had 2 REM episodes and 4 patients had only 1 REM episode. There was a negative correlation between the score on Epworth Sleepiness Scale (ESS) and the number of REM episodes (tab. 3).
Tab. 3. Spearman’s correlation coefficient between sleep characteristics, age and ESS
| Sleep characteristics | Parameters | Age | ESS |
| Total Sleep Time | Correlation Coefficient | -0.021 | 0.14 |
| Sig. (2-tailed) | 0.886 | 0.332 |
| N | 50 | 50 |
| Sleep latency | Correlation Coefficient | 0.267 | 0.054 |
| Sig. (2-tailed) | 0.061 | 0.708 |
| N | 50 | 50 |
| Sleep efficiency | Correlation Coefficient | -0.147 | -0.146 |
| Sig. (2-tailed) | 0.309 | 0.311 |
| N | 50 | 50 |
| REM episodes | Correlation Coefficient | -0.001 | -0.306* |
| Sig. (2-tailed) | 0.996 | 0.031 |
| N | 50 | 50 |
| REM sleep onset latency | Correlation Coefficient | 0.057 | 0.01 |
| Sig. (2-tailed) | 0.693 | 0.948 |
| N | 50 | 50 |
| PLMS index | Correlation Coefficient | -0.371** | -0.119 |
| Sig. (2-tailed) | 0.008 | 0.41 |
* statistically significant correlation (p < 0.05)
** statistically significant correlation (p < 0.01)
REM sleep onset latency was prolonged (> 2 hours) in 82% (n = 42) of our patients. Periodic limb movements in sleep (PLMS), i.e. repetitive, stereotyped movements in the NREM sleep (9), were seen in 18 patients. On the basis of the number of PLMS movements per hour of sleep, PLMS index is calculated. The upper limit for PLMS index is 5 (9). There was a negative correlation between age and PLMS index (tab. 3). In older patients (≥ 75 years of age), PLMS index was lower (tab. 2). All the patients in our study who had been diagnosed with restless leg syndrome (RLS) had PLMS. Sleep latency was prolonged in 7 out of 17 patients having RLS.
The classification of the severity of Sleep-Disordered Breathing (SDB) is based on the AHI score, with the score < 5 being classified as normal, 5-15 – mild, 15-30 – moderate, > 30 – severe SDB (6). 64% (n = 32) of our patients were diagnosed with OSAS (fig. 1). The prevalence of severe, moderate and mild OSAS was 22%, 32% and 10%, respectively. Moderate and severe OSAS (AHI > 15) patients had higher age than patients without OSAS (p < 0.005). The mean ESS score was higher in OSAS patients (p = 0.05). Snoring was present in 78% of the OSAS patients. Apnea witnessed by a partner was the most specific symptom, present in 92% of OSAS patients (fig. 2). We did not find significant differences between the groups with and without OSAS in regard of UPDRS and Hoehn & Yahr’s modified evaluation scale and Schwab & England’s functional evaluation scale.
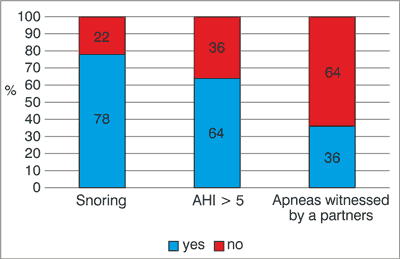
Fig. 1. The prevalence of snoring and OSAS
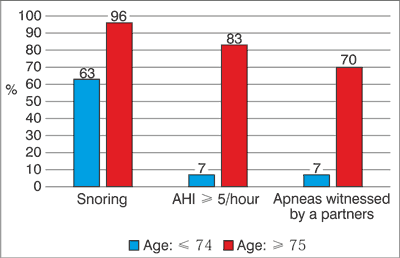
Fig. 2. Mild and severe OSAS by age
Discussion
Sleep disorders are common in Parkinson’s disease (PD). Nocturnal sleep disturbances and excessive day time somnolence are more frequent in patients with PD than in the healthy controls (10-12). The prevalence and the pattern of sleep disturbances are evaluated in this study.
Our observations are similar to what was reported in other papers (13-18). Our study showed PLMS in 18 patients (5.33 ± 1.03). Other studies reported PLMS of 22.02 ± 3.6 (14) and 55.4 ± 3.47 (12) in PD patients. 17 of our patients had RLS. All the patients in our study having RLS had PLMS (100%). 7 patients having RLS were found to have prolonged sleep latency. RLS also correlates with the duration of the disease and its severity, as well as with the sleep quality. In another study (19), it was reported that 20.8% of the PD patients had RLS, which was double the prevalence in a healthy control population.
Sleep Disordered Breathing (SDB) includes apnea and hypopnea. The etiology may be obstructive, central or mixed. The classification of the severity of SDB is based on AHI scoring, AHI > 5 being the criterion for diagnosing SDB. The most common type of SDB is obstructive sleep apnea (20). OSAS affects at least 2-66% of the general population (4, 10). Some studies have demonstrated that PD patients generally had a lower prevalence of OSAS. These results could be explained primarily by the lower BMI of PD patients when compared with the general population (3).
Sleep processes are directly and strongly correlated with the pathophysiological process of the PD. Some of the previous studies proposed that sleep and the motor centers are both affected in PD and the rate of degeneration of one is different from the other, and hence, sleep parameters do not correlate with the motor symptoms severity (21, 22).
Conclusions
OSAS is a common sleep disorder in PD patients. It has a higher prevalence in older PD patients and it is associated with greater daytime sleepiness. Snoring and apneas witnessed by a partner are the most common symptoms of OSAS in PD patients.
Piśmiennictwo
1. Barone P, Antonini A, Colosimo C et al.: The Priamo study: a multicenter assessment of nonmotor symptoms and their impaction quality of life in Parkinson’s disease. Movement Disorders 2009; 24: 1641-1649.
2. Lim SY, Lang AE: The nonmotor symptoms of Parkinson’s disease – an overview. Movement Disorders 2010; 25: S123-130.
3. Zeng J, Wei M, Li T et al.: Risk of Obstructive Sleep Apnea in Parkinson’s Disease: A Meta-Analysis. PLoS ONE 2013; 8: e82091.
4. Neto MS, Pereira M, Sobreira E et al.: Obstructive sleep apnea syndrome in Parkinson’s disease: preliminary results. Sleep 2013; 14: e112.
5. Hardy J, Gwinn-Hardy K: Genetic classification of primary neurodegenerative disease. Science 1998; 282: 1075-1079.
6. Flemons WW, Buysse D, Redline S et al.: Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999; 22: 667-689.
7. Wang YQ, Li R, Zhang MQ et al.: Neurobiological Mechanisms and Treatments of REM Sleep Disturbances in Depression. Curr Neuropharmacol 2015; 13: 543-553.
8. Weigand D, Michael L, Schulz H: When sleep is perceived as wakefulness: an experimental study on state perception during physiological sleep. J Sleep Res 2007; 16: 346-353.
9. Högl B: Periodic limb movements are associated with disturbed sleep. J Clin Sleep Med 2007; 3: 12-14.
10. Abrishami A, Khajehdehi A, Chung F: A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anesth 2010; 57: 423-438.
11. Ondo WG, Vuong KD, Khan H et al.: Daytime sleepiness and other sleep disorders in Parkinson’s diease. Neurology 2001; 57: 1392-1396.
12. Menza M, Dobkin RD, Marin H, Bienfait K.: Sleep Dysfunction in Parkinson’s Disease. Mov Disord 2010; 25: S117-122.
13. Diederich NJ, Vaillant M, Mancuso G et al.: Progressive sleep ‘destructuring’ in Parkinson’s disease. Sleep Med 2005; 6: 313-318.
14. Dhawan V, Healy G, Pal S, Chaudhuri KR: Sleep-related problems of Parkinson’s disease. Age Ageing 2006; 35: 220-228.
15. Kaynak D, Kiziltan G, Kaynak H et al.: Sleep and sleepiness in patients with Parkinson’s disease before and after dopaminergic treatment. Eur J Neurol 2005; 12: 199-207.
16. Wetter TC, Collado-Seidel V, Pollmacer T et al.: Sleep and periodic leg movement patterns in drug-free patient with Parkinson’s disease and multiple system atrophy. Sleep 2000; 23: 361-367.
17. Selvaraj VK, Keshavamurthy B: Sleep Dysfunction in Parkinson’s Disease. J Clin Diagn Res 2016; 10: OC09.
18. Covassin N, Neikrug AB, Liu L et al.: Clinical correlates of periodic limb movements in sleep in Parkinson’s disease. J Neurol Sci 2012; 316: 131-136.
19. Ondo WG: Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease. In: Videnovic A (ed.): Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease; 1st ed., Springer. Wien 2015: 159-171.
20. Arnulf I, Neutel D, Herlin B et al.: Sleepiness in Idiopathic REM Sleep Behavior Disorder and Parkinson Disease. Sleep 2014; 38: 1529-1535.
21. Joy SP, Sinha S, Pal PK et al.: Serial macro-architectural alterations with levodopa in Parkinson’s disease: Polysomnography (PSG)-based analysis. Ann Indian Acad Neurol 2015; 18: 309-313.
22. Breen DP, Vuono R, Nawarathna U et al.: Sleep and Circadian Rhythm Regulation in Early Parkinson Disease. JAMA Neurol 2014; 71: 589-595.

