Weronika Piotrowska1, Piotr Kwast2, *Lidia Zawadzka-Głos2
Gradenigo’s syndrome as a rare complication of acute otitis media in a child – case study
Zespół Gradenigo jako rzadkie powikłanie ostrego zapalenia ucha środkowego u dziecka – opis przypadku
1Students’ Scientific Group of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Students scientific group supervisor: Piotr Kwast, MD
2Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Zespół Gradenigo (GS) jest rzadkim powikłaniem ostrego zapalenia ucha środkowego (OZUŚ) i definiowany jest jako triada objawów, na którą składają się: jednostronne ropne zapalenie ucha środkowego, ból w obszarze zaopatrywanym przez nerw trójdzielny oraz niedowład nerwu odwodzącego. Częstości występowania tego powikłania wynosi mniej niż 2 na 100 tys. przypadków OZUŚ. Zakrzepica zatoki jamistej (CST) jest rzadkim powikłaniem występującym najczęściej po przebytym zapaleniu zatok przynosowych, opisano jednak kilka przypadków zakrzepicy w przebiegu GS.
Prezentujemy przypadek 11-letniego chłopca z wywiadem przebytego ostrego zapalenia ucha środkowego, skierowanego do Kliniki Otolaryngologii Dziecięcej z innego ośrodka z powodu epizodu wymiotów, bólu głowy, zaburzeń czucia prawej połowy twarzy oraz podwójnego widzenia. W konsultacji tomografii komputerowej (TK) opisano cechy zakrzepicy zatoki jamistej. Wykonany rezonans magnetyczny (MRI) twarzoczaszki z kontrastem wykazał stan zapalny w obrębie szczytu piramidy kości skroniowej prawej z ropniem podokostnowym oraz zakrzepicę zatoki jamistej po stronie prawej. Kontynuowano rozpoczętą dożylną antybiotykoterapię ceftriaksonem i metronidazolem, dołączono enoksaparynę oraz wankomycynę. Pacjenta zakwalifikowano do antromastoidektomii i drenażu ucha prawego. W 5. dobie po operacji włączono leczenie warfaryną. Po osiągnięciu terapeutycznego INR, odstawiono enoksaparynę i wypisano pacjenta do domu do dalszego leczenia ambulatoryjnego. Okres okołooperacyjny przebiegał bez powikłań, ustąpiły bóle głowy i zaburzenia czucia. W 11. tygodniu od wypisu w MRI nie stwierdzono cech ropnia podokostnowego ani zakrzepicy zatoki jamistej. Porażenie nerwu odwodzącego całkowicie ustąpiło.
Choć obecnie zarówno zespół Gradenigo, jak i zakrzepica zatoki jamistej stanowią rzadkie rozpoznania, znajomość możliwych manifestacji klinicznych tych powikłań jest istotna nie tylko dla laryngologów, ale również dla pediatrów oraz specjalistów medycyny rodzinnej. Wczesna diagnoza i rozpoczęcie leczenia są kluczowe w zapobieganiu groźnym dla życia konsekwencjom.
Summary
Gradenigo’s syndrome (GS) is a rare complication of otitis media and is defined as a concomitant unilateral acute inflammation of the middle ear, facial pain in the region supplied by the trigeminal nerve and abducens nerve palsy. It occurs in less than 2 in 100 thousand cases of acute otitis media. Cavernous sinus thrombosis (CST) is a rare complication usually accompanying sinusitis, however a few cases of CST in the course of GS were described before.
We present a case of a 11-year old boy with a history of otitis media admitted to the Department of Pediatric Otolaryngology of a tertiary care center with vomiting, headache, unilateral paresthesia of the face and diplopia. Cavernous sinus thrombosis as well as inflammation of the temporal bone including the petrous apex with formation of a subperiosteal abscess was shown in CT and MRI scans. The patient was treated with ceftriaxone, metronidazole and vancomycin, as well as enoxaparin and warfarin. Antromastoidectomy of the right temporal bone was performed. The patient recovered gradually over the course of treatment with a complete resolution of CST in MRI and abducens nerve palsy after in clinical examination after 11 weeks of treatment.
Even though both Gradenigo’s syndrome and cavernous sinus thrombosis are rare entities the knowledge of their clinical manifestations is very helpful for both laryngologists, pediatricians and general practitioners. Early diagnosis and prompt introduction of treatment are crucial for successful treatment and avoiding dangerous sequelae.
Introduction
Gradenigo’s syndrome (GS) was first described in 1907 by Guisseppe Gradenigo as the clinical triad of unilateral suppurative otitis media, pain in the region supplied by the trigeminal nerve, and ipsilateral abducens nerve palsy (1). The classical triad only occurs in less than half of patients (2).
Cavernous sinus thrombosis (CST) is a rare complication that usually arises from an infection involving the paranasal sinuses. Few case reports have been published describing venous sinus thrombosis associated with GS (3-5).
Although the incidence of GS and its potential complications have diminished since the advent of antibiotics, it is of utmost importance to remember about their life-threatening consequences that require immediate medical intervention (2, 6).
Case report
An 11-year-old boy with a history of acute otitis media (AOM) over the previous month was admitted to the Department of Pediatric Otolaryngology due to an episode of vomiting, headache, sensory disturbances in the right side of the face and diplopia.
The patient was transferred to the Department from another hospital, where computed tomography (CT) of the head was performed and intravenous treatment with ceftriaxone, metronidazole and dexamethasone was introduced.
On admission, the patient reported skin numbness in the right supraorbital area, and diplopia. Physical examination revealed impaired right eye abduction due to abducens nerve palsy (fig. 1a, b). The boy showed no symptoms related to other cranial nerve paresis. Meningeal signs were negative. Otoscopic examination revealed reddened right tympanic membrane without apparent pus in the middle ear (fig. 2).
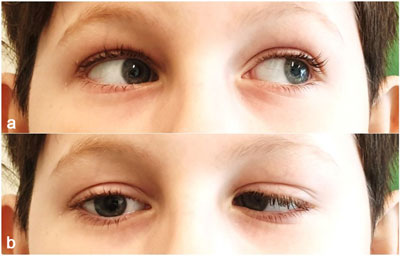
Fig. 1a, b. Right abduncens nerve palsy on admission when looking to the left (a) and right (b)
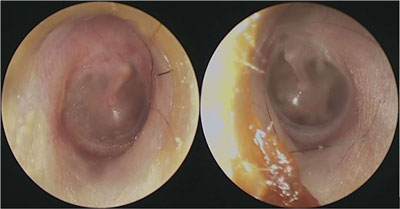
Fig. 2. Otoscopy on admission
Blood tests revealed slightly elevated white blood cell (WBC) count of 13.04 x 103/μL with 8.46 x 103/μL (64.8%) neutrophils. Renal and liver function as well as C-reactive protein were within normal limits. The values of D-dimers 606.04 μg/L FEU and fibrinogen 5.38 g/L were elevated.
Ophthalmological assessment confirmed right abducens nerve palsy, with normal fundoscopy, and normal visual acuity.
Radiological consultation of the previously made CT of the head showed signs of cavernous sinus thrombosis. A contrast-enhanced magnetic resonance imaging (MRI) of the head was performed, which showed inflammation of the right petrous apex with a subperiosteal abscess and cavernous sinus thrombosis on the right side (fig. 3).
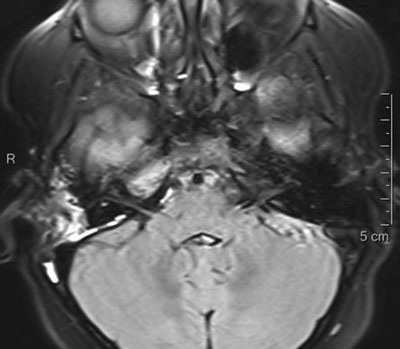
Fig. 3. MRI showing inflammatory changes in the temporal bone
The patient was diagnosed with GS based on his clinical presentation, which included history of acute otitis media, abducens nerve palsy, sensory disturbances in the distribution of the trigeminal nerve and the evidence of petrous apicitis. Imaging studies also revealed the presence of cavernous sinus thrombosis.
Previously started intravenous antibiotic therapy was continued, vancomycin and enoxaparin were also initiated. The patient was submitted to right antromastoidectomy with tympanostomy tube placement. Histopathological examination of the tissues removed during surgery showed remodeled, fibrotic inflammatory granulation tissue with perivascular inflammatory infiltrates. The postoperative period proceeded without complications. Headaches and sensory disturbances resolved. A gradual, albeit slow, improvement in the mobility of the right eye was observed. On the fifth day after surgery, warfarin treatment was started. After reaching the therapeutic INR range, enoxaparin was discontinued and the patient was discharged home for further outpatient treatment in good general condition, with the recommendation of a follow-up visit and right eye exercise according to ophthalmological recommendations.
In the follow-up contrast-enhanced cranial MRI performed at the 4th week after discharge, visible isolated minor thrombotic changes in the cavernous sinus were described, and outpatient treatment with warfarin was continued. In the 11th week after discharge, a fresh MRI scan showed a slightly increased signal intensity, absence of restricted diffusion, and a moderate contrast enhancement within the right petrous apex. There were no signs of a subperiosteal abscess or cavernous sinus thrombosis, which allowed for the termination of anticoagulant treatment. During the entire period of warfarin therapy, the patient did not experience any episodes of bleeding. The palsy of the abduction nerve resolved completely.
Discussion
Acute otitis media is the second most common pediatric diagnosis in the emergency department following upper respiratory infections. It is estimated that approximately 80% of children will experience a case of otitis media at least once in their lifetime (7). AOM complications are defined as the transition of the inflammatory process from the middle ear to the adjacent structures and are divided into intratemporal and intracranial complications (8). One of the most dangerous consequences of the spread of the disease is inflammation of the right petrous apex, which in the past occurred once in every 300 cases of otitis media and was associated with high mortality (2). The introduction of antibiotic therapy treatment reduced the incidence of this complication to less than 2 in 100,000 AOM cases (2).
The best known clinical manifestation of petrous apicitis (PA) is the triad of symptoms described by Guisseppe Gradenigo in 1907, which includes unilateral suppurative otitis media, pain in the distribution of the trigeminal nerve, often also referred to as retro-orbital pain, and ipsilateral abducens nerve palsy (1, 8). It should be remembered that the classic triad occurs only in about 14-42% of cases (2). The course of the disease in patients presenting a complete clinical picture and in patients who do not develop all components of the triad is similar (9).
The petrous part of the temporal bone has the shape of a three-sided pyramid with its apex directed medially and anteriorly (10). Close to the petrous apex, separated from it only by dura mater, lies the trigeminal ganglion (1). The abducens nerve passes through the Dorello canal located between the top of the petrous part of the temporal bone and under the petrosphenoidal ligament also known as the Gruber’s ligament (11). Due to anatomical proximity of those important neurological structures the inflammatory process can easily transition from the middle ear and cause the symptoms of GS (11). An important role in spreading the infection is played by pneumatization of the temporal bone pyramid, analogous to that in the mastoid process, which is present in approximately 30% of the population (2, 12, 13). Infection may spread to remote areas by direct extension and through vascular channels (12). The further development of the infection may give rise to other intracranial complications, such as meningitis, subperiosteal abscess, paresis of the IX, X, XI cranial nerves, or prevertebral or parapharyngeal abscess (1). In nonpneumatized mastoids spread of the infection may be caused by retrograde thrombophlebitis involving the petrosal sinuses especially the venules of the petrous apex (14). This may explain the rare cases of cavernous sinus thrombosis complicating PA (2-5).
Differential diagnosis of GS includes inflammatory pseudotumor, tuberculosis, sarcoidosis, Tolosa-Hunt syndrome, intracranial abscess, and malignancy (rhabdomyosarcoma, metastases) (15, 16). The final diagnosis is typically confirmed by imaging diagnostics. Each patient with suspected complications of AOM should undergo computed tomography (CT) and magnetic resonance imaging (MRI) as soon as possible. CT scans help identify air-cell opacification, trabecular breakdown, or erosion of cortical bone and is an important element in planning possible surgical treatment (3). MRI is more sensitive in detecting dural thickening and enhancement as well as intracranial complications and has the advantage of avoiding unnecessary radiation which is important in case of pediatric patients (3, 15).
Proper management of GS is difficult to establish due to the lack of specific guidelines and the rarity of this pathology. Some authors suggest the possibility of effective conservative treatment, while in severe cases, surgical intervention is often necessary. The decision on its initiation and range should be adjusted individually in each case (9, 16, 17). Broad-spectrum antibiotic therapy is recommended for all patients with GS and should include the most common pathogens causing AOM such as Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus spp., Pseudomonas spp., beta-hemolytic streptococci, and anaerobic bacteria (2, 7, 8). Most authors suggest the use of cephalosporin or penicillin, metronidazole, and vancomycin (2-5). When the disease is diagnosed in the advanced stage or if it does not improve during conservative treatment, surgical treatment is necessary (1, 2, 18, 19). Surgical access to the petrous part of the temporal bone is limited and carries a significant risk of permanent hearing damage (2, 20). Mastoidectomy with ventilation tube placement is a significantly safer method of surgical treatment that brings good results (3, 4, 9, 18-21). Considering the serious complications of radical surgery and the risk of dangerous consequences in the absence of effectiveness of conservative treatment alone, the choice of less invasive surgical treatment seems to be the most reasonable solution (21). However, the final choice of the surgical method depends on the pre-operative hearing condition of the patient, the severity of the disease, the individual anatomy of the temporal bone, and the surgeon’s experience (21).
The incidence of cavernous sinus thrombosis ranges from 0.2 to 1.6 per 100,000 people per year, with a slightly higher incidence in children than in adults (22). The most common cause of the disease is an infection located in the central area of the face, especially in the triangular area in between the corners of the mouth and the bridge of the nose (22). Few cases of cavernous sinus thrombosis in the course of GS have been described in the literature (3, 5, 19). Clinically, CST is manifested by fever and delirium, vomiting, and involvement of the cranial nerves crossing the sinus lumen (III, IV, V1, V2, VI) (22). The mortality caused by CST before the introduction of antibiotic therapy was as high as 80%, currently it remains at the level of 8-13% (22). The use of anticoagulation remains a controversial issue in the treatment of thrombotic complications associated with head and neck infections in children (23). A Cochrane review of anticoagulation for cerebral venous sinus thrombosis found a trend towards reduced death and disability in the anticoagulated group (24). These data concerned adults and did not reach statistical significance. Despite the lack of randomized controlled trials in pediatric patients, the benefits of anticoagulation appear to outweigh its risks (5, 6, 19, 23, 25).
Conclusions
Gradenigo’s syndrome is seldom seen in modern medicine thanks to widespread antibiotic use for the treatment of acute otitis media. Rare cases of the cavernous sinus thrombosis are a potentially life-threatening complications of GS. Each child with suspected complications after AOM should undergo a CT or MRI scan. The knowledge of possible clinical manifestations of rare AOM complications is important not only for specialists in otolaryngology, but also for pediatricians and family medicine specialists, because early recognition and timely management are crucial in preventing the occurrence of life-threatening consequences.
Piśmiennictwo
1. Motamed M, Kalan A: Gradenigo’s syndrome. Postgrad Med J 2000; 76(899): 559-560.
2. Gadre AK, Chole RA: The changing face of petrous apicitis – a 40-year experience. Laryngoscope 2018; 128(1): 195-201.
3. Sousa Menezes A, Ribeiro D, Balona F et al.: Gradenigo’s Syndrome with Carotid Septic Stenosis. Case Rep Otolaryngol 2020; 2020: 9439184.
4. Jensen PV, Hansen MS, Møller MN, Saunte JP: The Forgotten Syndrome? Four Cases of Gradenigo’s Syndrome and a Review of the Literature. Strabismus 2016; 24(1): 21-27.
5. Özkaçmaz S: Acute otitis media associated with Gradenigo syndrome and transverse sinus thrombosis: a case report. J Int Med Res 2019; 47(3): 1348-1352.
6. Sweis R, Biller J: Cavernous Sinus Thrombosis in Children. Pediatr Neurol Briefs 2016; 30(1): 4.
7. Danishyar A, Ashurst JV: Acute Otitis Media. [In:] StatPearls. Treasure Island (FL): StatPearls Publishing; March 16, 2021.
8. Leskinen K: Complications of acute otitis media in children. Curr Allergy Asthma Rep 2005; 5(4): 308-312.
9. McLaren J, Cohen MS, El Saleeby CM: How well do we know Gradenigo? A comprehensive literature review and proposal for novel diagnostic categories of Gradenigo’s syndrome. Int J Pediatr Otorhinolaryngol 2020; 132: 109942.
10. Bochenek A, Reicher M: Budowa szczegółowa czaszki. [W:] Leżańska T, Suchorska-Dubina N (red.): Anatomia człowieka. Tom I. Państwowy Zakład Wydawnictw Lekarskich, Warszawa 1990: 345.
11. Aleksandrowicz R, Ciszek B: Anatomia kliniczna głowy i szyi. Wydawnictwo Lekarskie PZWL, Warszawa 2007.
12. Allam AF, Schuknecht HF: Pathology of petrositis. Laryngoscope 1968; 78(11): 1813-1832.
13. Connor SE, Leung R, Natas S: Imaging of the petrous apex: a pictorial review. Br J Radiol 2008; 81(965): 427-435.
14. Gadre AK, Brodie HA, Fayad JN, O’Leary MJ: Venous channels of the petrous apex: their presence and clinical importance. Otolaryngol Head Neck Surg 1997; 116(2): 168-174.
15. Vazquez E, Castellote A, Piqueras J et al.: Imaging of complications of acute mastoiditis in children. Radiographics 2003; 23(2): 359-372.
16. Janjua N, Bajalan M, Potter S et al.: Multidisciplinary care of a paediatric patient with Gradenigo’s syndrome. BMJ Case Rep 2016; 2016: bcr2015214337.
17. Kazemi T: Acute Otitis Media-Induced Gradenigo Syndrome, a Dramatic Response to Intravenous Antibiotic. Iran J Otorhinolaryngol 2017; 29(92): 165-169.
18. Humayun HN, Akhtar S, Ahmed S: Gradenigo’s syndrome – surgical management in a child. J Pak Med Assoc 2011; 61(4): 393-394.
19. Heshin-Bekenstein M, Megged O, Peleg U et al.: Gradenigo’s syndrome: is fusobacterium different? Two cases and review of the literature. Int J Pediatr Otorhinolaryngol 2014; 78(1): 166-169.
20. Goldstein NA, Casselbrant ML, Bluestone CD, Kurs-Lasky M: Intratemporal complications of acute otitis media in infants and children. Otolaryngol Head Neck Surg 1998; 119(5): 444-454.
21. Demir B, Abuzaid G, Ergenc Z, Kepenekli E: Delayed diagnosed Gradenigo’s syndrome associated with acute otitis media. SAGE Open Med Case Rep 2020; 8: 2050313X20966119.
22. Plewa MC, Tadi P, Gupta M: Cavernous Sinus Thrombosis. [In:] StatPearls. Treasure Island (FL): StatPearls Publishing; September 29, 2021.
23. Rebelo J, Nayan S, Choong K et al.: To anticoagulate? Controversy in the management of thrombotic complications of head & neck infections. Int J Pediatr Otorhinolaryngol 2016; 88: 129-135.
24. Coutinho J, de Bruijn SF, Deveber G, Stam J: Anticoagulation for cerebral venous sinus thrombosis. Cochrane Database Syst Rev 2011; 2011(8): CD002005.
25. Geng B, Wu X, Malhotra A: Septic cavernous sinus thrombosis – case series and review of the literature. Clin Neurol Neurosurg 2020; 197: 106092.


