Experimental research into oedema of the brain, caused by some nervous factors, and indications for the administration of clenbuterol in some CNS disorders, particularly cerebral palsy
The presence of autonomic peripheral structures which are controlled through the truncus sympathicus is significant in the for structural organisation of the sympathetic nervous system. The sympathetic postganglionic fibres of the periarterial carotid plexus arise from the rostral segment of the sympathetic trunk, and in particular from the superior cervical ganglion. The final branching of carotid plexus takes place between adventitia and media (33), and supplies the brain as well as the hypophysis and pineal gland. An adrenergic neuro-transmitters, and probably endorphin peptide neurotransmitters, encephalines, and other substances secreted from the ends of sympathetic fibres regulate the permeability of the capillary endothelium (6), and the transport via the blood-brain barrier by means of astrocytes which connect the capillary walls together with neurocytes. Astrocytes show other functions as well, e.g. they absorb surplus K+ ions and supply neurocytes with glucose, prevent diffusion of neurotransmitters outside the synaptic fissure, etc. (25). An adrenergic neuro-transmitter from the ends of symphatic fibres reacts with the adrenergic receptors of the pinealocytes, and stimulates the synthesis of melatonin (30). It is not yet known whether peripheral impulses, which is the brain´s sympathetic stimulation coming from the external carotid plexus, predominates over central adrenergic stimulation, which has its source in the neurons of the locus coeruleus this forming a centre which supplies the whole brain (23).
In aqueous oedema (angiogenic and osmotic), the results of the disconnection of the internal carotid plexus have been observed along with the plexus´s reaction to nervous tissue under stimulation and/or anaesthesia (9). After craniotomy and incision of the dura mater, one can observe swollen brain tissue´s protruding from below the meninx, and take specimen of living brain tissue for analysis. A model of watery intoxication affected an experimental animal relatively slightly, (the majority managing to survive). The effects obtained were recurrent, and it was investigate some minor morphological changes in the capillaries and nervous tissue (phase-contrast microscopy) (figures 1-9).

Fig. 1. Rat´s cerebral cortex. Magn. appr. 1350 x.
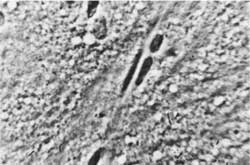
Fig. 2. Rat´s cerebral cortex: erythrocytes are visible in the capillaries, no signs of oedema. Magn. appr. 900 x.

Fig. 3. Normal rat´s brain cortex. Inside capillary erythrocytes are visible. In the capillary walls are endothelium cells. Magn. appr. 900 x.

Fig. 4. Border of cortex with white substance. On the left are visible oligodendrocytes and neurocytes on the right.

Fig. 5. Rat´s brain edema around capillaries. Encapsulated erythrocytes inside capillaries (stasis). Oedema fluid in brain substance. Magn. appr. 900 x.
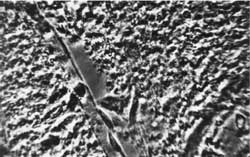
Fig. 6. Rat´s brain oedema. Greatly swollen astrocytes. Magn. appr. 1300 x.

Fig. 7. Rat´s brain oedema: perivascular fluid capsule. Magn. appr. 900 x.

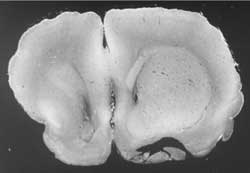
Fig. 8 & 9. Asymetrical brain oedema, more on the right, i.e. after truncus sympathicus blockade.
Both brain stimulation by means of strychnine and medicines which stimulate the sympathetic system (induced intrathecally or intraperitonealy), reduced swelling. The onset of oedema occured later on and was less prominent. Urethane anaesthesia contributed to the formation of swelling. Exclusion of the internal carotid plexus caused substantiall increase in swelling on that side. The microphotographs have not yet been published, but illustrate the following conclusions – "Sympathetic stimulation makes the dynamics of both growth and extent of oedema difficult, while its disconnection gives facilities for the dynamics of oedema”. This capability probably influences the metabolism of the nervous cells.
The blood-brain barrier and astrocytes which mediate between capillaries and neurocytes take part in the development of brain oedema. Conclusions quoted from a report dated 1965 are still current.
Ract et al. (35) demonstrated that lesion of the blood-brain barrier after a serious brain injury is related to a deficiency in catecholamines (dopamine and norepinephrine), and brings about intensified permeability of the capillaries. The neuroprotective action of adrenergic substances is connected with increased oxygen consumption of nervous tissue, blood flow and biochemical stimulation of brain tissue which ensures an increase in defensive properties this cells and decrease in oedema.
Glutamic acid, a basic stimulating transmitter, which appears in substantially after brain injury, can also produce an oedematogenic effect, since it leads to increased permeability of capillary endothelium, glia cells, and neurocytes. Glutamic acid can be found in quantity in cerebrospinal fluid from patients who suffer from acute viral meningitis, myelopathy, or acute disseminated sclerosis, that is to say all diseases accompanied by oedema (40).
Maxwell et al. (31) proved that swollen astrocytes, which limit glutamate diffusion from the site of an injury (traumatic oedema) protect neurocytes from swelling brought about by a surplus of glutamine irritation. In other words, astrocytes create a barrier which protects neurocytes from swelling.
A surplus of glutamine irritation is not advisable, as it may produce an toxic effect and cell necrosis. A surplus of catecholamine causes haemorrhage. Hiperammonaemia can lead to brain swelling after conquering the swollen astrocytes and serotoninergic activity (2) has been overcome.
Much research in recent years has indicated a progressing permeability of the blood-brain barrier, brain swelling during hypoxaemia, coma, poisoning, after brain injury. In such circumstances GABA-ergic stimulation prevails, and trofic adrenergic and glutaminergic1 stimulation are reduced.
Borges et al. (7) proved that the adrenergic central nervous system effectively reduced angiogenic brain swelling. In experiments are used beta2-adrenoceptor agonist, that is to say clenbuterol which inhibits the permeation of Evans´ blue and water through vessel walls and `saved´ (i.e. restrained) the development of brain swelling during the experiment.
The following results confirm the conclusions of an experiment carried out in 1965, others experiments emphasise a decrease in capillary permeability under the influence of adrenergic medicine, a specially beta2-adrenoreceptors, as proved in an vitro experiment by Allen and Coleman (1).
Beaumont et al. (5) revealed that, in increasing intracranial pressure, dopamine can normalize brain blood flow, but in arterial hypertension it raises the permeability of the blood-brain barrier, in contrast to epinephrine which lowers the permeability (42).
Researches by Ito et al. (24) on the morphology of swelling after an experimental injury revealed by MRI and diffusion-weighted magnetic resonance imaging (DWI), the substantial significance of arterial hypertension and hypoxaemia, which initially cause, the development of the cytotoxic swelling accompanied by a surplus of dopamine. Then it occurs to restriction of tissue mediators (endothelina, acidosis, nitrogen oxide (NO) (41). One has demonstrated (19) that all the factors mentioned together with glutamic acid have an impact on brain activity connected with regional blood flow (rCBF) which is of great significance to the functioning condition of the brain tissue´s connected with the modulation of neurotransmitters.
Glutamic acid – a stimulating neurotransmitter, as it is known, works in the opposite way from inhibitory transmitter, such as is to say gaba-aminobutyric acid (GABA). An increase of extracellular glutamate is characteristic of people with severe brain injury (44), and microglia is the restraining factor responsible for its concentration (29). Lowering the glutamate concentration and NaCl channel blocking decreases damage to the brain caused by oedema and can therefore give indication concerning cure. Taurine´s concentration is said to be an indicator of an induced swelling (in vitro experiment). The presence of taurine in cerebrospinal fluid may be useable as an indicator of the progress of therapy in cases of brain damage with swelling, caused by trauma (40).
Beta2-adrenomimetics, especially clenbuterol
Among numerous scientific experiments in the recent years, it has been revealed that clenbuterol, by way of blood, stimulates adrenergic receptors of transversely striped muscles, and in this way the mass of the muscles and their power can be increased, and neuropathic atrophy regressed and the CNS can be influenced (vide literature quoted in Kobel-Buys and Buys), (27). Clenbuterol shows an anticonvulsant effect(17) and corticosterone inhibits an adrenergic response of clenbuterol (10).
Propranolol – an antagonist of beta-adrenergic receptors - reduces clenbuterol´s neuroprotective effect, whereas a fall in propranolol restores clenbuterol´s activity. In the experiment this means that it works due to activation of the beta-adrenergic receptor (39).
Culmsee et al. (11) and Junker et al. (25) indicated in their experiments on rats that beta2- adrenomimetic (clenbuterol) is capable of protecting the brain from any damage caused by hypoxaemia, in portions of 0.01-0.5 mg/kg administrated peritoneally: it stimulates astrocytes, reduces the volume of cortical infarction and protects from the development of swelling. This neuroprotective effect takes place via sympathetic stimulation mediated by nerve growth factor (NGF) and other brain - derived neurotrophic factors such as BDNF, or neurotrophin 3 - 6 (NT 3 - 6). All these support neuron survival in the central nervous system.
These and other data lead to a hypothesis about therapy for the CNS illnesses and cerebral apoplexy by means of neuroprotective factors (clenbuterol included) (38).
Apart from clenbuterol, there are other neuroprotective chemical compounds – active choline, so called CDP - choline, a derivative from the combination of acetylocholine and citicoline which is a natural endogenic compound and shows neuroprotective effect in an experimental ischaemia of the brain (4).
Melatonin – is a highly efficacious, physiological hormone, "purifying” the brain of free radicals (antioxidants). Numerous experimental research projects have proved its neuroprotective effect. The report by Sarrafzadeh(36) is worthy of notice, referring to the mentioned interdependence between sympathetic system and pineal body.
NMDA´s receptor derives from N-metylo-D-asparaginic acid, and takes part in numerous aspects of functioning of the brain´s superior activities as well as in pathologies (30), shows a neuroprotective effect in focal ischaemia, and reduces brain oedema (28).
In the above report some possibilities for protective and trophic effects on CNS stimulating neurotransmitters and beta2-adrenomimetics, initially clenbuterol and then some other neuroprotective factors have been discovered. The results of experiments under consideration have pointed a direction for future researches which will probably be of great importance in the therapy of the nervous system.
The meaning of adrenergic neurotransmitters and others for focal nerve stimulation
The adrenergic neurons located in locus coeruleus make as mentioned above – a focus, which by means of adrenergic neurotransmitters and others makes possible bioelectric irritability in CNS and influences its activity. Noradrenaline (NA) and other monoaminmoergic transmitters increase nervous issue irritability, and function alongside GABA-ergic transmitters (inhibitive). Glutamic acid is first of all a binder of cortico-cortical connections (20). Inhibitive transmitters prevail during anaesthesia, coma etc. and function as a base for irritability - reducing medicine, e.g. anaesthetics, barbiturates and benzodiazepam (30).
Adrenergic-dopaminergic, serotonin and nicotine and other receptors, are found in the whole brain, and modulate functional systems, also brain-motorial. A deficiency of nicotine receptors in basal nuclei takes place in Alzheimer´s disease (26). In image examinations a dopamine deficit appears significantly, e.g. in Parkinson´s disease (34).
Modulation is essential for the brain´s functioning at the time of being sick or healthy and it creates a signs of a) changes in the concentration of neurotransmitters, b) it is connected with hesitation in the metabolism of the nervous tissue, and c) regulation of the brain´s regional blood flow.
These changes may be examined when a person is healthy or suffers from mental anxiety, Alzheimer´s disease, depression, schizophrenia, memory disturbances (13), motor disturbances (e.g. dystonia, restless leg´s syndrome, motor hyperexcitability), dyslexia and others by means of visualising methods, which allow an image of metabolic and regional blood flow in various parts of the brain by means of positron tomography (PET) and fMRI (12, 14, 20, 34).
Changes in monoaminergic neurotransmitter concentration and others that cause variable motor behaviour were noticed during experimental research; after prefrontal dopaminergic blockade, adrenergic receptors showed hyperactivity and give rise to abnormal motor stereotypes, motor hyperactivity and a type of anxiety like response (15). In other similar experiments they caused motor stereotypes and motor expressions which depended on dopaminergic hyperactivity or a surplus of noradrenergic or adrenaline or metanefryne (16).
Possibility of beta2-adrenomimetic administration in people, in particular using clenbuterol in both peripheral and central nervous system disturbances
It has been known for a long time that clenbuterol has a peripheral effect on the smooth muscles of the bronchi, and therefore it is significant in asthma treatment, including in children.
Clenbuterol´s influence on the muscles and the peripheral nervous system are expressed by means of muscle cell protection after their denervation, and peripheral nerve regeneration, which has been confirmed in the last two years by Frerichs et al. (21, 22). A slight advantage has been taken of its effect therapy for illnesses connected with peripheral muscles and nerves.
Floßdorf et al. (18) applied clenbuterol successfully in people (without any fundamental justification - „ut aliquid fiat”) in cases of muscular atrophy caused by inactivity, and in neuromuscular diseases and other illnesses of the nervous system. Schreiber et al. (37) used clenbuterol in various types of congenital myopathy and observed an improvement. It is however necessary to carry out a further study with the application of a statistical methods in order to state the degree of objective improvement.
A publication on the administration of beta2-adrenomimetic in adults appeared recently, showing that they raise L-dopa and L-leucyne (43) concentrations and therefore they soothe symptoms of Parkinson´s disease and make it possible to reduce a daily dose of L-dopa (40). We are still waiting for further results based on a relatively large group of patients and the results of treatment of other neuro-degenerative illnesses.
Justification of clenbuterol administration in cerebral palsy
During the perinatal period, after brain damage, mental retardation often appears (or a psychological aberration without dysfunction of any intelligence as well as paresis, extrapyramidal disorders, epilepsy, motor anxiety and others. The anxiety is visible in the form of spasmodic twitching, stereotypes, various motor habits, running, throwing objects, etc. Motor hyperirritability is connected not only with brain injuries and mental retardation, but also with the absence of natural parents or exposure to alcoholism or to persecution. Motor hyperirritability may be reduced in adults (32). Therefore, for proper pharmacological treatment aiming at a reduction in hyperirritability being brought up, in a good atmosphere and with good living standards are indispensable.
Motor hyperirritability, as mentioned, is connected with disintegration of the brain´s functions caused by improper modulation of neurotransmitters, which creates a reason for imperfect control over both psychological and motor activities.
In growing child clenbuterol has a trophic impact on CNS and peripheral nervous system. This impact creates the chance of an improvement in muscle metabolism, subsidence of muscular atrophy (of muscle, neurogenic, or central origin), an improvement in nervous tissue metabolism mediated by adrenergic neurotransmitters, which may reach to proper modulation of functional programes. A reduction in motor hyperirritability might take place, and thereafter paresis or ataxia.
The above experimental research on brain oedema which dependent on some nervous factors, in particular on adrenergic neurotransmitters have been brought forward, and the research on neuroprotective factors have justified a hypothesis concerning degenerative and vascular illnesses of the central nervous system, treated by means of clenbuterol, and the possibility of clenbuterol application in some illnesses of the CNS and peripheral nervous system in children.
After obtaining permission from DIL (The Medical Council) Bioethic Commission´s in Wrocław (resolution num. 44, 2000) a research work entitled "Finding grounds for clenbuterol medical treatment of some diseases of CNS at children suffering from cerebral palsy” was carried out at my management in Rehabilitation Centre for Disabled Children in Mikoszów/Strzelin. The research schedule was realized and worked on both clinically and statistically by Kobel-Buys and Buys (27). They have stated that clenbuterol significantly reduces psychomotor hyperirritability in mentally retarded children, and can improve kinetic ability in paresis. Extrapyramidal symptoms have not undergone any improvement after use of clenbuterol, and have even shown a transient increase in two ill children. Afterwards, use of clenbuterol was suspended in a hyperkinetic syndrome. The children reacted positively to the medicine, and side effects (tachycardia, anxiety, shaking hands and other examples in literature), were very slight. After the symptoms had been noticed by their parents a curing dose was reduced and the symptoms subsided following reduction of the dose after 2-3 days. Thus, they were practically no obstacle during the therapy.
It turned out that this well - known medicine in asthma therapy, used in both children and adults without any side effects, can be applied to children under treatment for disturbances of the CNS, especially in motor hyperirritability and paresis.
Therapy by means of clenbuterol is still being carried out, in particular for children and teenagers showing symptoms of motor hyperirritability or paresis. It has been noticed that the symptoms decrease. The results of therapy after 2000 (27) of approximately 120 patients have already been taken into consideration for the next publication.
1 On the margin on experimental water intoxication, we must mention 13 young women in whom after the drug "Ecstasy” and water intake, brain oedema and acute hyponatremia developed (3, 8).
Piśmiennictwo
1.Allen M.J., Coleman R.A.: Beta2-adrenoceptors mediate a reduction in endothelial permeability in vitro. Eur. J. Pharmacol. 1995, 14, 274, 1-3, 7-15.2.Bachmann C.: Mechanisms of hyperammonaemia. Clin. Chem. Lab. Med., 2002 40, 7, 633-62.3.Balmelli C. et al.: Fatal brain oedema after ingestion of ecstasy and benzylpiperazine, Dtsch. Med. Wochenchr. 2001,126. 28-29, 809-811.4.Baskaya M.K. et al.: Neuroprotective effects of citicoline on brain oedema and blood-brain barrier breakdown after traumatic brain injury: J Neurosurg 2000, 92, 3, 448-52.5.Beaumont A. et al.: Contrasting effects of dopamine therapy in experimental brain injury. J. Neurotrauma, 2001, 18, 12, 1359-72.6.Borges N. et al.: Changes in brain microvessel endothelial cell monolayer permeability induced by adrenergic drugs, Eur. J. Pharmacol., 1994, 269, 2.7.Borges N. et al.: Dynamicts of experimental vasogenic brain oedema in the rat: changes induced by adrenergic drugs. J. Auton. Pharmacol., 1999, 19, 4, 209-17.8.Brabach L., Humble M.: Young women die of water intoxication after taking one tablet of ecstasy. Today´s drug panorama calls for increased vigilance in health care. Lakartidingen 2001, 98, 8, 817-9.9.Brzecki A.: Der Einfluss der symphatischen Innervation und der Erregbarkeit des zentralen Nervensystems auf das experimentelle Hirnoedem. Proceedings Tom IV. 8 th International Congress of Neurology, Vienna, 5-10. IX., 1965. 357-360.10.Bugajski J. et al.: A single corticosterone pretreatment. inhibits the hypothalamic-pituitary adrenal responses to adrenergic and cholinergic stimulation J. Phys Pharmac. 2001, 52, 2, 313-24.11.Culmsee C. et al.: Clenbuterol induces growth factor mRNA, activates astrocytes, protects rat brain tissue against ischaemic damage. Eur. J. Pharmacol.,1999, 20, 379, 1, 33-43.12.Dolan R. et al.: Human Memory Systems, 381, Human Brain Function, Edits.: Frackowiak R. S. Friston K., J., Frith C., D., Dolan R., J., Mazziotta J., C. Academic Press, San Diego-Toronto, 1997.13.Dolan R.J. et al.: Measuring Neuromodulation with Functional Imaging. Human Brain Function, 404 – 428, Edit. Frąckowiak, Friston K.J., Frith C.D., Dolan R., J., Mazziota J.C., Acaemic Press, San Diego-Toronto, 1997.14.Eidelberg D: Functional brain networks in movement disorders. Current Opinion in Neurology, 1998, 11, 319-326.15. Espejo E.F., Minano J.: Adrenergic hyperactivity and metanephrine excess in the nucleus accumbens after prefrontocortical dopamine depletion. J. Neurophysiol 2001, 85, 30-4.16.Espejo E.F. et al.: Behavioural expression of opiate withdrawal is altered after prefrontocortical dopamine depletion in rats: monoaminergic correlates. Neuropsychopharmacology, 2001, 25, 2, 204-12.17.Fisher W. et al.: Anticonvulsant and sodium channel blocking activity of higher doses of clenuterol. Arch. Pharmacol. (Namynyn Schmiedebergs) 2001, 363, 2, 182-192.18.Floßdorf W. et al.: Klenbuterol w zanikach mięśniowych i niedowładach różnego pochodzenia. MMW (edycja polska, 1998, 12, 29-32.19.Forman S.D. et al.: Simultaneous glutamate and perfusion fMRI responses to regional brain stimulation: J Cereb Blood Flow Metab 1998, 18, 10, 1064-70.20.Frąckowiak R.S.: The Cerebral Basis of Functional Recovery. 243 264, Human Brain Function., Edit Frąckowiak R. S., Frith Ch., D., Dolan R., J., Mazziota J., C., Academic Press, San Diego – Toronto, 1997.21.Frerichs O. et al.: The Influence on Nerve Regeneration by the beta2-receptor agonist Clenbuterol. Handschr. Mikrochir. Plast. Chir. 2002, 34, 2, 84-8.22.Frerichs O. et al.: Regeneration of peripheral nerves after clenbuterol treatment in a rat model. Muscle Nerve 2001, 24, 12, 1087-91.23.Hanaway J. et al.: The Brain Atlas, Fitzgerald Science Press, Bethesda, Meryland, 1998.24.Ito J. et al.: Characterisation of oedema by diffusion-weighted imaging in experimental traumatic brain injury. J. Neurosurg. 1996, 84, 1, 97-103.25.Junker V. et al.: beta-adrenoceptors activate astrocytes and provide neuroprotection. Eur. J. Pharmacol. 2002, 20, 446, 1-3, 25-36.26.Kenn W.R.: The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer´s disease. Behaviour. Brain Res., 2000, 113, 1-2, 169-81.27.Kobel-Buys K., Buys G.: Wpływ podawania clenbuterolu na ośrodkowe zaburzenia ruchowe u dzieci chorych na mózgowe porażenie dziecięce, Pediatria, Current Medical Literature, 2000, 2, 82-90.28.Kroppenstedt S.N. et al.: Neuroprotective properties of aptiganel HCL (Cerestat) following controlled cortical impact injury. Acta Neurochir Suppl (Wien) 1998, 71, 114-6.29.van Landeghem F.K. et al.: Early expression of glutamate transporter proteins in ramified microglia after controlled cortical impact injury in the rat. Glia 2001, 35, 3, 167-79.30.Longstaff A.: Neurobiologia, przekład pod red. A. Wróbla, PWN Warszawa, 2002.31.Maxwell W.L. et al.: Massive astrocytic swelling in response to extracellular glutamate a possible mechanism for post-traumatic brain swelling? Acta Neurochir Suppl (Wien) 1994, 60, 465-7.32.Menkes J.H.: Textbook of Child Neurology. Williams & Wilkins, Baltimore – Tokyo, 1995.33.Müller L.R.: Lebensnerven und Lebenstriebe. Julius Springer, Berlin, 1931.34.Passingham R.: Functional Organisation of the Motor System Human Brain Function 243-269, Edit. Frąckowiak R.S., Friston K.J., Frith C.D., Dolan R.J., Mazziota J.C.: Academic Press, San Diego – Toronto, 1997.35.Ract C. et al.: Comparison of dopamine and norepinephrine after traumatic brain injury and hypoxic-hypotensive insult. J Neurotrauma 2001, 18, 11, 1247-54.36.Sarrafzadeh A.S. et al.: Neuroprotective effect of melatonin on cortical impact injury in the rat. Acta Neurochir (Wien) 2000; 1, 42, 11, 1293-9.37.Schreiber G. et al.: Clenbuterolbehandlung bei kongenitalen Myopathien: eine offene, nicht kontrollierte Pilotstudie, cyt. poz. 18.38.Semkova I, Krieglstein J.: Neuroprotection mediated via neurotrophic factor´s and induction of neurotrophic factors. Brain Res. Rev. 1999, 30, 2, 176-188.39.Semkova I. et al.: Clenbuterol protects mouse cerebral cortex and rat hippocampus from ischaemic damage and attenuates glutamate neurotoxicity in cultured hippocampal neurons by induction of NGF. Brain Res., 1996, 22, 717, 1-2, 44-54.40.Stover J.F. et al.: Neurotransmitters in cerebral fluid reflect pathological activity. Eur. J. Clin. Invest., 1998, 28, 9, 760-1.41.Thomale U. et al.: Cortical hypoperfusion precedes hyperperfusion following controlled cortical impact injury. Acta Neurochir. Suppl 2002, 81, 229-31.42.Tuor U. et al.: The effects of hypertension induced by catecholamines and the relationship between cerebral blood flow and glucose use. Am. J. Physiol., 1986, 251, 4, 2.43.Uc E.Y. et al.: Beta-adrenergics enhance brain extraction of levodopa. Mov. Disord. 2002, 17, 1, 54-9.44.Vespa P. et al.: Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J. Neurosurg 1998, 89, 69,71-82.
Pozostałe artykuły z numeru 1/2003:








