© Borgis - Postępy Nauk Medycznych 3/2017, s. 151-156
*Rafał Muc1, Agnieszka Saracen1, Jarosław Pinkas2, 3, Iwona Grabska-Liberek3
Diabetic macular edema management – international guidelines overview
Zarządzanie cukrzycowym obrzękiem plamki żółtej – przegląd międzynarodowych wytycznych
1Faculty of Health Sciences and Physical Education, K. Pulaski University of Technology and Humanities in Radom
Head of Faculty: Associate Professor Zbigniew Kotwica, MD, PhD
2Department of Health Care Organisation and Medical Certification, Centre of Postgraduate Medical Education, Warsaw
Head of Department: Jarosław Pinkas, MD, PhD
3Department of Ophthalmology, Centre of Postgraduate Medical Education, Prof. W. Orłowski Hospital, Warsaw
Head of Department: Professor Iwona Grabska-Liberek, MD, PhD
Streszczenie
Cukrzycowy obrzęk plamki żółtej (DME) jest ciężką chorobą, związaną z retinopatią cukrzycową (DR). Wszyscy pacjenci z cukrzycą są narażeni na ryzyko rozwoju DME. Nasilenie choroby może wahać się od stanu łagodnego do umiarkowanego i ciężkiego, z ryzykiem utraty wzroku. W ostatnich latach na całym świecie zostały opracowane wytyczne dotyczące zarządzania DME, w związku z czym istnieje konieczność dokonania przeglądu tych zaleceń, by móc znaleźć najlepszą opcję terapeutyczną dla pacjentów z cukrzycowym obrzękiem plamki żółtej.W niniejszym artykule autorzy, opierając się na międzynarodowych i polskich zaleceniach klinicznych, podsumowują obecne kierunki leczenia cukrzycowego obrzęku plamki żółtej. Badania i wnioski w tym artykule są oparte na oficjalnie dostępnych danych internetowych, opublikowanych wytycznych leczenia DME takich organizacji jak: Royal College of Ophthalmologists (Wielka Brytania), Canadian Diabetes Association (Kanada), American Association of Ophthalmologists (USA), International Council of Ophthalmology (USA) i Polskiego Towarzystwa Okulistycznego. Omawiane w artykule rekomendacje zostały dokładnie przeanalizowane i podsumowane. Wytyczne American Association of Ophthalmologists i International Council of Ophthalmology są najbardziej wyczerpujące pod kątem zapisów, jak należy zarządzać DME. Wydaje się, iż stanowią one najbardziej kompleksowe i zaawansowane podejście, które opiera się na medycynie opartej na faktach (Evidence Based Medicine). Panel ekspertów Polskiego Towarzystwa Okulistycznego opracował własne wytyczne zarządzania cukrzycowym obrzękiem plamki żółtej. Dokument ten jest oparty na badaniach międzynarodowych i jest zgodny z głównym nurtem międzynarodowych zaleceń leczenia DME. Zawiera on również stosunkowo innowacyjne podejście w stosowaniu preparatów anty-VEGF w leczeniu DME.
Celem tego artykułu było podsumowanie obecnych opcji zarządzania cukrzycowym obrzękiem plamki żółtej na podstawie uznanych międzynarodowych rekomendacji. Choć wśród analizowanych treści nie zidentyfikowano istotnych różnic w zarządzaniu DME, to niektóre akcenty różnicujące typu jaki typ leczenia należy zastosować i kiedy, są widoczne. Taka różnorodność w podejściu powinna być zawsze rozpatrywana przez okulistów przy poszukiwaniu najlepszej opcji terapeutycznej dla konkretnego pacjenta z DME.
Summary
Diabetic Macular Edema (DME) is a severe disease, related to Diabetic Retinopathy (DR). All diabetic patients are at risk of DME development. The disease severity may vary from mild to moderate and severe, with risk of loss of vision. In the last years, DME management guidelines have been developed worldwide, and there is necessity to review these recommendations, to find the best therapeutic option for Diabetic Macular Edema Patients.
In this article authors summarize what are the current Diabetic Macular Edema management options based on the international and Polish clinical recommendations and guidelines.
The study and conclusions in this article are based on web-available data, officially published DME management guidelines of Royal College of Ophthalmologists (UK), Canadian Diabetes Association, American Association of Ophthalmologists, International Council of Ophthalmology and Polish Ophthalmology Society.
The guidelines have been thoroughly reviewed and summarized in this article. Guidelines of American Association of Ophthalmologists as well as International Council of Ophthalmology are the most advanced in detailed description of DME management and seems to represent the most comprehensive and advanced approach, based on the evidence-based medicine. Polish Ophthalmology Society panel of experts has developed its own guidance of Diabetic Macular Edema management. However the document is based on international studies and is aligned with mainstream international recommendations, it contains also a novel approach of anti-VEGF usage in DME management.
This study aimed to show what are the current Diabetic Macular Edema management options based on the clinical recommendations and guidelines. However no substantial differences have been identified amongst reviewed guidelines, but some specific to a guideline accents of what treatment alternative should be used and on what stage of DME are visible. These diversities should be considered by ophthalmologists always when looking for the targeted therapeutic option to a specific DME patient.

Introduction
Diabetic Macular Edema (DME) is a severe disease, related to the Diabetic Retinopathy (DR). All diabetic patients are at risk of DME development. The disease severity may vary from mild to moderate, with risk of loss of vision. 25-30% non-ophthalmology treated, and up to 15% ophthalmology treated diabetic patients might be affected by moderate loss of vision due to DME (1). Based on the Rohit Varma, Neil M. Dressler study published in JAMA Ophthalmology weighted DME prevalence in USA is 3.8% (2.7-4.9%) of diabetes (2), however the meta-analysis of 35 studies (22,896 patients from United States, Australia, Europe and Asia) calculates DME prevalence on 7.48% (7.39-7.57) of the overall diabetes population (3).
Progression to DME affects 3% of mild non-proliferative DR eyes, 38% moderate and severe non-proliferative DR eyes and relates up to 71% eyes of the proliferative Diabetic Retinopathy – the most vision-threatening form of the disease (4, 5).
According to the Los Angeles Latino Eye Study and in the Proyecto VER study – 18% of participants with diabetes of more than 15 years’ duration had the proliferative DR, with no PDR percentage difference between type 1 vs type 2 diabetes (6, 7).
Polish National Health Fund (NFZ) estimates diabetes patients on 2 million in Poland (8). Based on NFZ data and referring to cited above Rohit Varma as well as Yau et al. studies, authors calculate DME prevalence from 76.000 to 149.000 patients in Poland (2, 3).
On the authority of Wilkinson et al. Diabetic Macular Edema is classified to mild – where some retinal thickening or hard exudates in posterior pole but outlying from the center of the macula, moderate where retinal thickening or hard exudates approaching the center of the macula but not involving the center and severe, where retinal thickening or hard exudates involving the center of the macula (9).
Based on selected international and Polish guidelines published in years 2012-2016 authors analyze what are the clinical recommendations in vision loss prevention amongst Diabetic Macular Edema patients, what are the current treatment options and what are the perspectives for the future.
The study and conclusions in this article are based on web-available data, officially published DME management guidelines of Royal College of Ophthalmologists (RCO, UK) – publication in year 2012, Canadian Diabetes Association (CDA, CAN) – publication in year 2013, Polish Ophthalmology Society (POS, PL) – publication in year 2014, American Association of Ophthalmologists (AAO, US) – last publication in year 2016 and International Council of Ophthalmology – first publication in year 2013, the latest publication in year 2016 (ICO, US).
The guidelines have been methodically read and key DME management tactics: screening of the patients, the disease assessment and treatment introduction have been summarized and compared one to another.
Discussion
In December 2012 the Royal College of Ophthalmologists, United Kingdom, published ‘Diabetic Retinopathy Guidelines’ (10) where Diabetic Retinopathy, including Diabetic Macular Edema management is precisely described.
Risk factors such as non-modifiable: genetic components, gender and duration of mellitus patients and modifiable: glycemia, blood pressure and lipid levels are considered as those which are playing main role for DR and DME development. Also carotid arterial disease, pregnancy, renal impairment and smoking should be taken into account in the complex disease management process (10).
According to the RCO recommendations, maintaining proper parameters of modifiable DR risk factors through effective treatment of primary diseases, have significant positive impact on long-term outcome of retinopathy. In the paper these have received ‘Level 1’ evidence (which is based on results of randomized controlled trials – RCTs) and mostly ‘Level A’ recommendations (where strength of evidence was universally agreed) (10).
Irrespectively of DR risk factors management strategies, RCO identifies four main therapeutic options of the DME treatment. These are laser photocoagulation, intravitreal steroid treatment, intravitreal VEGF inhibitors and polytherapy of laser photocoagulation + VEGF or + intravitreal steroid treatment (10).
RCO also considers vitrectomy for removal of hard exudates and for non-ischaemic diffuse DME when grid laser treatment does not bring expected results, but the evidence is based on case studies (10).
In year 2013 the Canadian Diabetes Association issued clinical practice guidelines (11) where experts committee emphasizes the necessity of mellitus patients screening, type 1 diabetes, all individuals ≥ 15 age, 5 years after diagnosis, and in all type 2 diabetes at diagnosis. If retinopathy is detected, then sight-threatening DR treatment should be introduced. Monitoring of the disease progress to be continued at least once per year. If DR is not present, then re-screening rhythm should be assigned, annually in all type 1 diabetes and type 2 diabetes every 1-2 years (11).
Despite direct DR treatment, CDA also recommends proper control of glycaemia, blood pressure and lipids to ‘reach targets per guidelines’, as these impact factors play an important role of retinopathy development. However anti-platelet therapy with ASA seems to be not associated with DR progression (11).
American Association of Ophthalmologists (AAO) published in year 2016 an updated preferred practice pattern (PPP) guidelines of Diabetic Retinopathy management (12).
AAO PPP guidelines are reviewed by panel of experts on an annual basis, and either no commercial financial support to these guidelines nor authors or reviewers received financial compensation for their work on the paper (12).
Key findings highlighted by AAO refer to necessity of type 1 and type 2 diabetes screening for DR and continuous controlling of glucose, blood pressure and serum lipids as abnormal levels of these parameters have significant impact on DR progression. All diagnosed non-proliferative, proliferative DR and diabetic macular edema patients must be referred to ophthalmologists. Concomitantly to CDA guidelines, AAO recommends follow up of DME progression at annual basis, irrespectively to type of mellitus (12).
As specified in the AAO guidelines, DR and DME diagnosis physical examination include primary disease history assessment like duration of diabetes, glycaemia and lipids levels, systemic hypertension, renal disease, obesity, ocular history and medicaments taken. Ocular examination should comprise from visual acuity assessment, intraocular pressure, pupillary assessment, funduscopy including examination of the posterior pole and examination of the peripheral retina and vitreous (12). Additional tests like color and red-free fundus photography, optical coherence tomography, fluorescein angiography and ultrasonography might enhance physical examination, and treatment outcomes follow up (12).
In year 2013 the International Council of Ophthalmology (ICO) released first time guidelines for Diabetic Eye Care. The ICO diabetic eye care committee consists from international experts of ophthalmology from the North and South Americas, Asia, Australia and Europe. The guidelines are updated on a regular basis and the newest release is elaborated for year 2017 (13). The ICO classifies diabetic eyes as having no Diabetic Macular Edema, non-central involved DME and central-involved DME (tab. 1) (13).
Tab. 1. Classification of Diabetic Macular Edema according to ICO (13)
| Diabetic Macular Edema | Observations on dilated ophthalmoscopy |
| No DME | No retinal thickening or hard exudates in the macula |
| Noncentral-involved DME | Retinal thickening in the macula that does not involve the central subfield zone that is 1 mm in diameter |
| Central-involved DME | Retinal thickening in the macula that does involve the central subfield zone that is 1 mm in diameter |
An excellent tool for grading Diabetic Retinopathy including Diabetic Macular Edema is available online at www.drgrading.iehu.unimelb.edu.au.
For non-central involved DME, ICO experts recommend the disease progress assessment every 3 months, for central involved DME every 1 month (tab. 2).
Tab. 2. DME progress assessment according to ICO (13)
| Classification of DME | Next screening schedule | Referral to Ophthalmologist |
| Noncentral-involved DME | 3 months | required |
| Central-involved DME | 1 month | required |
According to ICO guidelines, proper DR and DME management includes screening of the patients, detailed ophthalmic assessment and treatment of DME (fig. 1) (13).
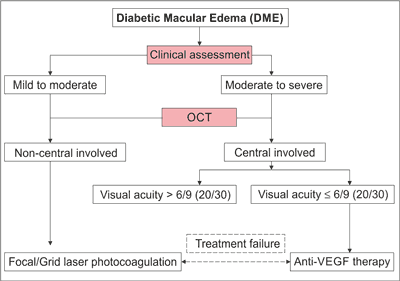
Fig. 1. DME treatment decision tree as per ICO (13)
Screening of the patients comprises of complete ophthalmic examination, including an identification of DR. While the detailed ophthalmic assessment covers (13):
– patient medical history with key elements like duration of mellitus, glycemia and ocular history, medicaments taken and other systemic diseases presence,
– physical exam through visual acuity assessment, measurement of intraocular pressure, gonioscopy when indicated, slit-lamp biomicroscopy and fundus examination,
– follow up examination comprising of follow up history, follow up physical exam, ancillary tests (optic coherence tomography – OCT, fundus photography, fluorescein angiography) and patients education.
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Mavrikakis E, Khan BU, Lam W-C: Macular Edema in Diabetes. Medscape 2016; http://emedicine.medscape.com/article/1224138-overview#a6 (accessed August 13, 2016).
2. Varma R, Bressler N, Doan QV et al.: Prevalence of and Risk Factors for Diabetic Macular Edema in the United States. JAMA Ophthalmol 2014; 132(11): 1334-1340; http://archopht.jamanetwork.com/article.aspx?articleid=1895297 (accessed August 13, 2016).
3. Yau JW, Rogers SL, Kawasaki R et al.: Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012; 35(3): 556-564; http://care.diabetesjournals.org/content/35/3/556 (accessed August 13, 2016).
4. Coscas G, Cuhna-Vaz J, Loewenstein A, Soubrane G: Macluar edema: a practical approach. Dev Ophthalmol 2010; 47.
5. Javadzadeh A: The effect of posterior subtenon methylprednisolone acetate in the refractory diabetic macular edema: a prospective nonrandomized interventional case series. BMC Ophthalmology 2006; 6: 15-19.
6. Varma R, Torres M, Pena F et al.: Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology 2004; 111(7): 1298-1306.
7. West SK, Klein R, Rodriguez J et al.: Diabetes and diabetic retinopathy in a Mexican-American population: Proyecto VER. Diabetes Care 2001; 24(7): 1204-1209.
8. Based on the NFZ data; http://nfz.gov.pl/nfz-blizej-pacjenta/cukrzyca/ (accessed: August 20, 2016).
9. Wilkinson CP, Ferris FL III, Klein RE et al.: Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003; 110(9): 1677-1682.
10. Ghanchi F, Bailey C, Chakravarthy U et al.: Diabetic Retinopathy Guidelines. The Royal College of Ophthalmologists, London 2012: 42-125; http://rcophth.ac.uk/wp-content/uploads/2014/12/2013-SCI-301-FINAL-DR-GUIDELINES-DEC-2012-updated-July-2013.pdf (accessed: December 28, 2016).
11. Boyd SR, Advani A, Altomare F et al.: Retinopathy. Can J Diabetes 2013; suppl. 1: 137-140; http://canadianjournalofdiabetes.com/article/S1499-2671(13)00039-7/pdf (accessed: January 2, 2017).
12. Olsen TW, Adelman RA, Flaxel CJ et al.: Retina/Vitreous Panel. Preferred Practice Patter® Guidelines. Diabetic Retinopathy. American Academy of Ophthalmology, San Francisco, CA 2016: 2-19; https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2016 (accessed: January 6, 2017).
13. Taylor H, Binder S, Das T et al.: Guidelines for Diabetic Eye Care. International Council of Ophthalmology, San Francisco, CA 2017: 7-25; http://www.icoph.org/downloads/ICOGuidelinesforDiabeticEyeCare.pdf (accessed: January 16, 2017).
14. Caldwell R, Bartoli M, Behzadian M et al.: Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives; Diabetes Metab Res Rev 2004; 19(6): 442-455.
15. Stewart MW, Rosenfeld PJ: Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 2008; 92: 667-668.
16. Do D, Nguyen Q, Shah S et al.: An exploratory study of the safety, tolerability and bioactivity of a single intravitreal injection of vascular endothelial growth factor Trap-Eye in patients with diabetic macular oedema. Br J Ophthalmol 2009; 93: 144-149.
17. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial Diabetic Retinopathy Vitrectomy Study. Ophthalmology 1988; 95(10): 1307-1320.
18. Czupryniak L, Dorecka M, Grabska-Liberek I: Zasady postępowania w cukrzycowym obrzęku plamki – wytyczne Polskiego Towarzystwa Okulistycznego 2014: 4-6; http://www.mp.pl/okulistyka/wytyczne/wytyczne-i-artykuly/103189,zasady-postepowania-w-cukrzycowym-obrzeku-plamki-wytyczne-pto-2014 (accessed: February 6, 2017).
19. Charakterystyka Produkty Leczniczego EYLEA; http://www.ema.europa.eu/docs/pl_PL/document_library/EPAR_-_Product_Information/human/002392/WC500135815.pdf (accessed: February 6, 2017).
20. Pinkas J, Muc R, Grabska-Liberek I: Diabetic Macular Edema Treatment Limits. Journal of Health Policy 2016; 1: 47-52; http://www.jhpor.com (accessed: February 7, 2017).a

