© Borgis - Postępy Nauk Medycznych 11/2016, s. 836-840
*Szymon Kawecki1, Mieszko Kozikowski1, Jakub Dobruch1, 2
The role of multiparametric magnetic resonance imaging (mpMRI) in the diagnosis of prostate cancer
Znaczenie wieloparametrycznego rezonansu magnetycznego (mpMRI) w rozpoznawaniu raka gruczołu krokowego
11st Unit of Didactics, Department of Urology, Centre of Postgraduate Medical Education, European Health Centre Otwock
Head of Department: Jakub Dobruch, MD, PhD
22nd Unit of Didactics, Department of Urology, Centre of Postgraduate Medical Education, Professor W. Orłowski Independent Public Teaching Hospital, Warsaw
Head of Department: Jakub Dobruch, MD, PhD
Streszczenie
Rak stercza jest drugim co do częstości nowotworem wśród mężczyzn w Polsce. Skomplikowana patofizjologia i przebieg choroby sprawiają, że niektóre metody obrazowania sprawdzają się lepiej od innych w poszczególnych stadiach klinicznych raka stercza. Wieloparametryczny rezonans magnetyczny dostarcza złożonej informacji na temat morfologii i zmian czynnościowych w sterczu, co powoduje, że jest on przydatny w wielu sytuacjach, w których standardowe metody obrazowania stają się niewystarczające. Praca stanowi przegląd aktualnych metod obrazowania raka stercza ze szczególnym naciskiem na znaczenie wieloparametrycznego rezonansu magnetycznego.
Summary
Prostate cancer is the second most common neoplasm in men in Poland. Due to the complex pathophysiology and the course of disease, some imaging methods prove more effective than others in certain clinical stages of prostate cancer. Multiparametric magnetic resonance imaging provides complex information about morphology and functional changes in the prostate, therefore it is useful in many situations, where standard imaging methods are insufficient. The paper is a review of current imaging modalities in prostate cancer, with particular emphasis on the significance of multiparametric magnetic resonance imaging.

Prostate cancer (PCa) is one of the most common malignancies in men. The incidence of PCa varies among different countries, with the highest rates in Australia, New Zealand, Northern America, particularly among black males, and Western Europe (fig. 1). The lowest rates are reported in Asian countries. An estimated 1.1 million men worldwide were diagnosed with PCa in 2012, accounting for 15% of all malignancies diagnosed in men at that time (1). In Poland, prostate cancer is the second most common neoplasm after lung cancer. About 10,201 men were diagnosed with PCa in 2012 in Poland (2).
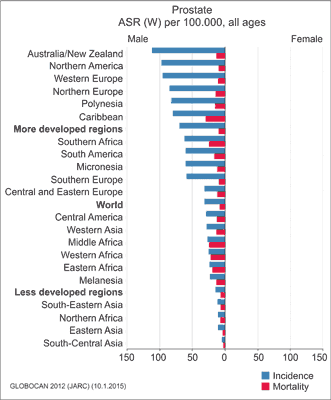
Fig. 1. Standardised incidence and mortality rates of prostate cancer in selected countries according to the Globocan database (WHO, 2012, www-dep.iarc.fr/globocan/globocan.html) (1)
PCa primarily develops in the peripheral zone of the prostate (fig. 2).
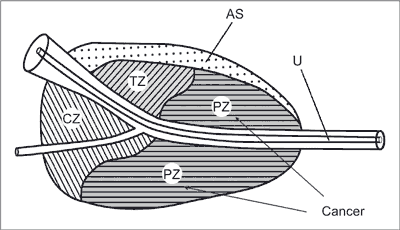
Fig. 2. The zonal structure of the prostate
AS – anterior commissure; TZ – transitional zone; CZ – central zone; PZ – peripheral zone; U – urethra (3)
Improved imaging methods for prostate scanning with enhanced biopsy accuracy for PCa diagnosis resulted in higher detection rates for tumours arising in the transition zone (TZ), located anterior to the urethra.
Initially, the cancer is limited to the prostate gland (organ-confined disease). However, with time it invades the periprostatic tissues (ECE – extraprostatic extension; locally advanced disease). Perineural invasion, i.e. tumour spread along a nerve, is a characteristic feature of PCa. Further development of PCa may result in the spread of cancer cells beyond the prostate gland, particularly to sites penetrated by the cavernous nerves, as well as lead to the invasion of the seminal vesicles as a result of cancer spread along the ejaculatory ducts, direct invasion of the seminal vesicles by the tumour itself or, least often, as a result of metastasis. The spread of cancer beyond the prostate gland can result in the invasion of the neck and the triangular region of the bladder. Ureteral invasion prevents the urine flow from the upper urinary tract, causing hydronephrosis, which may lead to renal failure. Anterior rectal wall invasion is rare as this part is protected against invasion by a strong structure known as the Denonvilliers fascia. Significant local progression of the primary tumour is usually accompanied by the presence of lymph node and distant metastases, bone metastases in particular.
Prostate-confined cancer is usually asymptomatic or causes scarce, non-specific symptoms. In some cases, clinical consequences of significantly advanced cancer, e.g. pain in bones, anaemia and/or renal insufficiency, are the first manifestations of PCa.
Transrectal ultrasound guided TRU-CUT core biopsy (TRUSTRU-CUT) of the prostate gland is the primary PCa diagnostic method. Indications for the TRU-CUT needle biopsy include abnormalities identified based on digital rectal examination (DRE), such as a nodular thickening in the prostatic parenchyma, generally increased prostate cohesion, asymmetry, blurred lateral boundaries and increased levels of serum prostate-specific antigen as well as lesions suspicious of PCa, which are detected by transrectal ultrasound (TRUS) or, recently increasingly often, by multiparametric magnetic resonance imaging (mpMRI). Prostate assessment based on DRE is very subjective and dependent on the experience of the examiner. TRUS-guided biopsy is superior in some respects when compared with DRE. It allows for an accurate determination of the size, boundaries and the internal structure of the prostate gland. Therefore, it is widely used for TRUSTRU-CUT. An hypoechoic area is a typical TRUS appearance of prostate cancer. Although about 50% of pathomorphologically confirmed prostate cancer foci are hypoechoic, the ultrasonographic pictures of poorly-differentiated cancers and cancers located outside the peripheral zone, where tumours most often develop, tend to vary (4, 5).
Clinical assessment of local tumour progression is based on DRE, TRUS, biopsy and, in some cases, computed tomography (CT) of the pelvis and/or mpMRI imaging. The mpMRI imaging technology is currently a method that most accurately reflects the structure of the prostate gland. This diagnostic method has been significantly improved in recent years. Currently, three-Tesla MRI scanners are available, which have better resolution of the obtained images, including dynamic contrast-enhanced images, compared to previous 1.5 Tesla MRI scanners. In addition to conventional morphology images, imaging using spectroscopy (magnetic resonance spectroscopic imaging – MRSI) and imaging based on water diffusion (diffusion-weighted MRI – DWMRI) have been introduced. Although collecting information obtained using all these technologies is very difficult, experience shows that combining at least two of them significantly improves the accuracy of evaluation, allowing for a final determination of the stage and the size of PCa. This information is useful in determining the extent of surgery (radical prostatectomy – RP) to minimise the risk of positive surgical margins (PSM) with the lowest possible impairment of quality of life.
MpMRI technology contributes to the improved diagnosis of PCa, which is of value for cancer patients at different stages of disease and in different clinical situations.
1. Localisation of PCa foci in patients with no abnormalities revealed by TRUS
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. GLOBOCAN 2012: Estimated cancer incidence, Mortality and Prevalence Worldwide in 2012; http://globocan.iarc.fr/.
2. Didkowska J, Wojciechowska U, Tarkowski W et al.: Nowotwory złośliwe w Polsce w 2003 roku. Centrum Onkologii – Instytut im. M. Skłodowskiej-Curie. Krajowy Rejestr Nowotworów, Warszawa 2006.
3. McNeal JE: The prostate and prostatic urethra: a morphologic synthesis. J Urol 1972; 107: 1008-1016.
4. Ahmed MM, Oesterling JE: Presentation and diagnosis. [In:] Hamdy FC, Basler JW, Neal DE, Catalona WJ (eds.): Management of urologic malignancies. Churchill Livingstone, London 2002: 139-150.
5. Borówka A, Chłosta P, Antoniewicz A et al.: Ultrasonografia w urologii. Ultrasonografia przezodbytnicza stercza – aspekty kliniczne i praktyczne. Ultrasonografia 2004; 16: 17-28.
6. Smith JA Jr, Scardino PT, Resnick MI et al.: Transrectal ultrasound versus digital rectal examination for the staging of carcinoma of the prostate: results of a prospective, multi-institutional trial. J Urol 1997; 157: 902-906.
7. Bratan F, Niaf E, Melodelima C et al.: Influence of imaging and histological factors on prostate cancer detection and localisation on multiparametric MRI: a prospective study. Eur Radiol 2013; 23: 2019-2029.
8. Hoeks CM, Schouten MG, Bomers JG et al.: Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol 2012; 62: 902-909.
9. Yuen JS, Thng CH, Tan PH et al.: Endorectal magnetic resonance imaging and spectroscopy for the detection of tumor foci in men with prior negative transrectal ultrasound prostate biopsy. J Urol 2004; 171: 1482-1486.
10. Tacikowska M: Ocena skuteczności badania MR w przedoperacyjnym określeniu stopnia zaawansowania raka gruczołu krokowego w korelacji z transrektalnym badaniem ultrasonograficznym. Nowotwory 2000; 50: 357-361.
11. Schnall MD, Connick T, Hayes CE et al.: MR imaging of the pelvis with an endorectal-external multicoil array. J Magn Reson Imaging 1992; 2: 229-232.
12. Park BH, Jeon HG, Jeong BC et al.: Influence of magnetic resonance imaging in the decision to preserve or resect neurovascular bundles at robotic assisted laparoscopic radical prostatectomy. J Urol 2014; 192: 82-88.
13. Xylinas E, Yates DR, Renard-Penna R et al.: Role of pelvic phased array magnetic resonance imaging in staging of prostate cancer specifically in patients diagnosed with clinically locally advanced tumours by digital rectal examination. World J Urol 2013; 31: 881-886.
14. Tiguert R, Gheiler EL, Tefilli MV et al.: Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology 1999; 53: 367-371.
15. May F, Treumann T, Dettmar P et al.: Limited value of endorectal magnetic resonance imaging and transrectal ultrasonography in the staging of clinically localized prostate cancer. BJU Int 2001; 87: 66-69.
16. Golimbu M, Morales P, Al-Askari S, Shulman Y: CAT scanning in staging of prostatic cancer. Urology 1981; 18: 305-308.
17. Hricak H, Dooms GC, Jeffrey RB et al.: Prostatic carcinoma: staging by clinical assessment, CT, and MR imaging. Radiology 1987; 162: 331-336.
18. Abuzallouf S, Dayes I, Lukka H: Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol 2004; 171: 2122-2127.
19. Harisinghani MG, Barentsz J, Hahn PF et al.: Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003; 348: 2491-2499.
20. McGregor B, Tulloch AG, Quinlan MF, Lovegrove F: The role of bone scanning in the assessment of prostatic carcinoma. Br J Urol 1978; 50: 178-181.
21. O’Donoghue EP, Constable AR, Sherwood T et al.: Bone scanning and plasma phosphatases in carcinoma of the prostate. Br J Urol 1978; 50: 172-177.
22. Gerber G, Chodak GW: Assessment of value of routine bone scans in patients with newly diagnosed prostate cancer. Urology 1991; 37: 418-422.
23. Terris MK, Klonecke AS, McDougall IR, Stamey TA: Utilization of bone scans in conjunction with prostate-specific antigen levels in the surveillance for recurrence of adenocarcinoma after radical prostatectomy. J Nucl Med 1991; 32: 1713-1737.
24. Buell U, Kleinhans E, Zorn-Bopp E et al.: A comparison of bone imaging with Tc-99m DPD and Tc-99m MDP: concise communication. J Nucl Med 1982; 23: 214-217.
25. Paulson DF, the Uro-Oncology Research Group of current staging procedures in assessing disease extent of prostatic adenocarcinoma. J Urol 1979; 121: 300-302.
26. Umbehr MH, Muntener M, Hany T et al.: The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 2013; 64: 106-117.
27. Heidenreich A, Bastian PJ, Bellmunt J et al.: EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 2014; 65: 467-479.
28. Evangelista L, Briganti A, Fanti S et al.: New Clinical Indications for (18)F/(11)C-choline, New Tracers for Positron Emission Tomography and a Promising Hybrid Device for Prostate Cancer Staging: A Systematic Review of the Literature. Eur Urol 2016; 70: 161-175.
29. Perera M, Papa N, Christidis D et al.: Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016 Jun 27. pii: S0302-2838(16)30293-7.
30. Cirillo S, Petracchini M, Scotti L et al.: Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur Radiol 2009; 19: 761-769.
31. Liauw SL, Pitroda SP, Eggener SE et al.: Evaluation of the prostate bed for local recurrence after radical prostatectomy using endorectal magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2013; 85: 378-384.


