Prostate carcinoma (PCa) is one of the most common cancers in men both in terms of incidence and mortality in Poland and worldwide (1).
Prostate cancer predominantly develops (70%) in the peripheral zone of the prostate gland. Approx. 10-15% of PCas are found in the transitional zone, and 15-20% in the central zone (2).
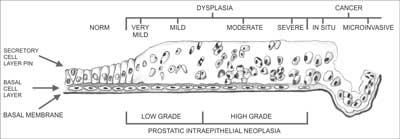
Carcinogenesis within the prostate gland is a biologically complex phenomenon. It typically begins with precancerous changes in the epithelium and progresses to invasive cancer. The processes commonly associated with precancerous changes or co-existing with cancer include prostatic intraepithelial neoplasia (PIN) and typical small acinar proliferation (ASAP). Originally, three PIN forms were recognized, differing by the degree of cellular abnormality and the percentage of abnormal epithelial cells: PIN 1 (benign), PIN 2 (moderate) and PIN 3 (severe) (3). Currently, just 2 PIN types are differentiated, namely low grade and high grade (4). Low grade prostatic intraepithelial neoplasia (LGPIN) has been determined to differ in nature from high grade prostatic intraepithelial neoplasia (HGPIN), as it is associated with cancer only in isolated cases (5, 6), and does not constitute a separate pathomorphological entity (7). PIN is diagnosed based on multiple clearly defined architectural and cytological criteria (8). Several features make PIN a precancerous change. It occurs in the prostate in the fourth and fifth decade of life, and its prevalence grows with age. PIN precedes PCa by a minimum of 5-10 years (9). It is found in approx. 60-90% of PCas and is frequently situated near (< 2 mm) invasive cancer site (5, 9-12). As opposed to PCa, PIN retains an intact or fragmented basal cell layer, hence its presence is not associated with elevated PSA level in blood serum. The percentage of HGPIN found in needle prostate biopsy without coexisting PCa ranges from 0.15-16% (5, 13-20). Prevalence of PCa identified in repeat biopsy of a gland where previously HGPIN was found ranges from 22-100% (5, 6, 18-23).
Atypical small acinar proliferation (ASAP) is a pathological change of the prostatic epithelium that may be indicative of PCa. ASAP consists in the presence of foci of small, atypical glands suspicious for cancer, yet not referred to as cancerous, since their basal membrane is preserved (24). The percentage of ASAP diagnosed in needle biopsy of the prostate gland, without coexistence of cancer, ranges from 1.5-6.3% (5, 13-20), whereas the prevalence of PCa identified in a follow-up biopsy of a prostate gland where ASAP was previously detected ranges from 22-100% (5, 6, 18-23).
Owing to the different morphology of the changes listed above, it should be remembered that when a follow-up prostatic biopsy is conducted, the location of cancerous tissue may differ from the location of previously identified HGPIN, whereas every repeat prostatic biopsy of a gland where ASAP was previously identified should heavily focus on the areas where it was found (21, 23, 25, 26). The process of carcinogenesis (fig. 1) involves mutation of cells in the afore mentioned precancerous changes, which, in turn, leads to dysplastic changes resulting with carcinoma in situ (CIS), and ultimately with invasive carcinoma. The majority of prostatic cancers are multifocal. The multiple foci typically arise in various prostatic zones and are characterized by varying histological grades (27-29).
A crucial element of tissue core examination is identifying their malignancy score. Malignancy is defined according to the rules developed by Donald Gleason (1920-2008) that he originally described in 1966 in the journal “Cancer Chemotherapy Report”, in his paper that had previously been rejected by two major urological journals (30-32). PCa grading according to Gleason scale is at present a universally used method of PCa evaluation.
As a young pathologist, Donald Gleason (fig. 2) worked on the histological interpretation of prostatic cancer at the Minneapolis Veterans Administration Medical Center from 1962. He based his study on the results of 280 prostatic biopsies performed between 1960-1964 (33). The scale he proposed has been in common use since 1978, when it was adopted by the American Cancer Society and approved by the WHO (33, 34).
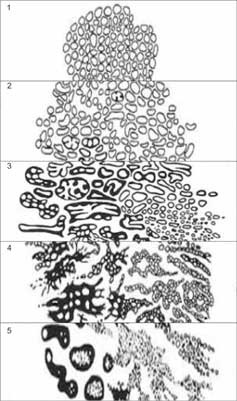
Gleason scale recognizes 5 PCA malignancy patterns, ranging from 1 (least malignant) to 5 (most malignant) differing predominantly by the architectural features, and, to a lesser degree, by the appearance of the cancerous cells (fig. 3) (35).
1. onkologia.org.pl.
2. Theodorescu D: Prostate cancer, clinical oncology. [In:] Schwab M (ed.): Encyclopedic Reference of Cancer. 1st ed., vol. 720. Springer Verlag, New York 2001.
3. McNeal JE, Bostwick DG: Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol 1986; 17: 64-71.
4. Haggman MJ, Macoska JA, Wojno KJ, Oesterling JE: The relationship between prostatic intraepithelial neoplasia and prostate cancer: critical issues. J Urol 1997; 158: 12-22.
5. Keetch DW, Humphrey P, Stahl D et al.: Morphometric analysis and clinical follow-up of isolated prostatic intraepithelial neoplasia in needle biopsy of the prostate. J Urol 1995; 154: 347-351.
6. Davidson D, Bostwick DG, Qian J et al.: Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol 1995; 154: 1295-1299.
7. Epstein JI, Grignon DJ, Humphrey PA et al.: Interobserver reproducibility in the diagnosis of prostatic intraepithelial neoplasia. Am J Surg Pathol 1995; 19: 873-886.
8. Foster CS, Cornford P, Forsyth L, Djamgoz MBA: The cellular and molecular basis of prostate cancer. British Journal of Urology 1999; 83: 171-194.
9. Sakr WA, Haas GP, Cassin BJ et al.: Frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol 1993; 150: 379-385.
10. Qian J, Wollan P, Bostwick DG: The extent and multicentricity of high-grade prostatic intraepithelial neoplasia inclinically localized prostatic adenocarcinoma. Hum Pathol 1997; 28: 143-148.
11. Wiley EL, Davidson P, McIntire DD, Sagalowsky AI: Risk of concurrent prostate cancer in cystoprostatectomy specimens is related to volume of high-grade prostatic intraepithelialneoplasia. Urology 1997; 49: 692-696.
12. Sakr WA, Grignon DJ: Prostatic intraepithelial neoplasia and atypical adenomatous hyperplasia. Relationship to pathological parameters, volume and spatial distribution of carcinoma of the prostate. Anal Quant Cytol Histol 1998; 20: 417-423.
13. Cheville JC, Reznicek MJ, Bostwick DG: The focus of “atypical glands, suspicious for malignancy” in prostatic needle biopsy specimens. Am J Clin Pathol 1997; 108: 633-640.
14. Weinstein MH, Greenspan DL, Epstein JI: Diagnoses rendered on prostate needle biopsy in community hospitals. Prostate 1998; 35: 50-55.
15. Bostwick DG, Qian J, Frankel K: The incidence of high grade prostatic intraepithelial neoplasia in needle biopsies. J Urol 1995; 154: 1791-1794.
16. Ouyang RC, Kenwright DN, Nacey JN, Delahunt B: The presence of atypical small acinar proliferations in prostate needle biopsy is predictive of carcinoma on subsequent biopsy. BJU Int 2001; 87: 70-74.
17. Chan TY, Epstein JI: Follow-up of atypical prostate needle biopsies suspicious for cancer. Urology 1999; 53: 351-355.
18. Khan W, Sakr WA, Grignon DJ et al.: Analysis of 3300 consecutive prostate biopsies: a 4 year institutional experience with the frequency of carcinoma, isolated high-grade intraepithelial neoplasia, and “atypical” (Abstract). Mod Pathol 1997; 10: 79.
19. O’Dowd GJ, Miller MG, Orozco R, Veltri RW: Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology 2000; 55: 553-559.
20. Wills ML, Hamper UM, Partin AW, Epstein JI: Incidence of high-grade prostatic intraepithelial neoplasia in sextant needle biopsy specimens. Urology 1997; 49: 367-373.
21. Kamoi K, Troncoso P, Babaian RJ: Strategy for repeated biopsy in patients with high grade prostatic intraepithelial neoplasia. J Urol 2000; 163: 819-823.
22. Ellis WJ, Brawer MK: Repeat biopsy: who needs it? J Urol 1995; 153: 1496-1498.
23. Shephard D, Keetch DW, Humphrey PA et al.: Repeat biopsy strategy in men with isolated prostatic intraepithelial neoplasia of prostate needle biopsy. J Urol 1996; 156: 460-463.
24. Rosai J: Surgical Pathology. Elsevier 2004; 18: 1372-1381.
25. Allen EA, Kahane H, Epstein JI: Repeat biopsy strategies for men with atypical diagnoses on initial prostate needle biopsy. Urology 1998; 52: 803-807.
26. Langer JE, Rovner ES, Coleman BG et al.: Strategy for repeat biopsy of patients with prostatic intraepithelial neoplasia detected by prostate needle biopsy. J Urol 1996; 155: 228-231.
27. Miller GJ, Torkko KC: Natural history of prostate cancer – epidemiologic considerations. Epidemiol Rev 2001; 23: 14-18.
28. Byar DP, Mostofi FK: Carcinoma of the prostate: prognostic evaluation of certain pathologic features in 208 radical prostatectomies: examined by the step-section technique. Cancer 1972; 30: 5-13.
29. Miller GJ, Cygan JM: Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol 1994; 152(5 Pt. 2): 1709-1713.
30. Gleason DF: Classification of prostatic carcinomas. Cancer Chem Rep 1966; 50: 125-128.
31. Gleason DF, Mellinger GT: Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974; 111: 58-64.
32. Gleason DF: Histologic Grading of Prostate Cancer. Human Pathology 1992; 3 (23): 273-279.
33. Schmidt C: Gleason Scoring system Faces Change and Debate. JNCI J Natl Cancer Inst 2009; 101(9): 622-629.
34. Phillips JL, Sinha AA: Patterns, Art, and Context: Donald Floyd Gleason and the Development o the Gleason Grading System. Urology 2009; 74(3): 497-503.
35. Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974; 111: 58-64.
36. Montironi R, Mazzuccheli R, Scarpelli M et al.: Gleason grading of prostate cancer in needle biopsies or radical prostatectomy specimens: contemporary approach, current clinical significance and sources of pathology discrepancies. BJUInt 2005; 95: 1146-1152.
37. Carlson GD, Calvanese CB, Kahane H, Epstein JI: Accuracy of biopsy Gleason scores from a large uropathology laboratory: use of a diagnostic protocol to minimize observer variability. Urology 1998; 51: 525-529.
38. Brimo F, Schultz L, Epstain J: The value of mandatory second opinion pathology review of prostate needle biopsy interpretation before radical prostatectomy. J Urol 2010; 184: 126-130.
39. Melia J, Moseley R, Ball RY et al.: A UK based investigation of inter- and intra- observer reproducibility o Gleason grading o prostatic biopsies. Histopathology 2006 May; 48(6): 644-654.
40. Griffiths DFR, Melia J, McWilliam LJU et al.: A study of Gleason score interpretation in different groups o UK pathologists; techniques for improving reproducibility. Histopathology 2006; 48: 655-662.
41. William C, Allsbrook WC Jr, Johnson MH et al.: Interobserver reproducibility of Gleason grading o prostatic carcinoma: general pathologists. Hum Pathol 2001; 32: 81-88.
42. Svanholom H, Mygind H: Prostatic carcinoma. Reproducibility o histologic grading. Acta Path Microbiol Immunol Scand 1985; 93: 67-71.
43. Ozdamar SO, Sarikaya S, Yildiz L et al.: Intraobserver and interobserver reproducibility o WHO and Gleason histologic grading system in prostatic adenocarcinomas. Int Urol Nephrol 1996; 28: 73-77.
44. McLean M, Srigley J, Banerjee D et al.: Interobserver variation in prostate cancer scoring: are there implications for the design of clinical trials and treatment strategies? Clin Oncol 1997; 9: 222-225.
45. di Loreto C, Fitzpatrick B, Underhill S et al.: Correlation between visual clues, objective histologic features, and interobservere agreement in prostate cancer. Am J Clin Pathol 1991; 96: 70-75.
46. Bain GO, Koch M, Hanson J: Feasibility of grading carcinomas. Arch Pathol Lab Med 1982; 106: 265-267.
47. de las Morenas A, Siroky MB, Merriam J et al.: Prostatic adenocarcinoma: reproducibility and correlation with clinical stages o four grading systems. Hum Pathol 1988; 19: 595-597.
48. Epstein JL, Allsbroock WC Jr, Amin MB, Egevad LL: ISUP grading committee. The 2005 International Society of Urologic Pathology (ISUP) Consensus Conference on Gleason trading of Prostatic Carcinoma. Am J Surg Pathol 2005; 29: 1228-1242.
49. Montironi R, Vela-Navarrete R, Lopez-Beltran A et al.: 2005 update on pathology of prostate cancer. EU 2006; 49: 441-447.
50. Billis A, Guimaraes MS, Freitas LLL et al.: The Impact of the 2005 International Society o Urological Pathology Concensus Conference on Standard Gleason Grading o Prostatic Carcinoma on Needle Biopsies. J Urol 2008; 4(18): 548-553.
51. Vos EK, Litjens GJ, Kobus T: Assessment of Prostate Cancer Aggressiveness Using Dynamic Contrast-enhanced Magnetic Resonance Imaging at 3 T. Eur Urol 2013 Sep; 64(3): 448-455.
52. Masterson TA, Touijer K: The role of endorectal coil MRI in preoperative staging and decision-making for the treatment of clinically localized prostate cancer. MAGMA 2008 Nov; 21(6): 371-377.
53. Beyersdorff D, Darsow U, Stephan C: MRI of prostate cancer using tree different coil systems: image quality, tumor detection and staging. Rofo 2003 Jun; 175(6): 799-805.
54. Hoeks CM, Hambrock T, Yakar D et al.: Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 2013 Jan; 266(1): 207-217.
55. Lemaitre L, Puech P, Poncelet E et al.: Dynamic contrast-enhanced MRI of anterior prostate cancer: morphometric assessment and correlation with radical prostatectomy findings. Eur Radiol 2009 Feb; 19(2): 470-480.
56. Borofsky MS, Rosenkrantz AB, Abraham N et al.: Does Suspicion of Prostate Cancer on Integrated T2 and diffusion-weighted MRI Predict More Adverse Pathology on Radical Prostatectomy? Urology 2013; 81(6): 1279-1283.
57. Oto A, Yang C, Kayhan A et al.: Diffusion-Weighted and Dynamic Contrast-Enhanced MRI of Prostate Cancer: Correlation of Quantitative MR Parameters With Gleason Score and Tumor Angiogenesis. AJR Am J Roentgenol 2011 Dec; 197(6): 1382-1390.
58. Epstein JI, Egevad L, Amin MB et al.; Grading Committee: The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Pathol 2016 Feb; 40(2): 244-252.