Monika Jabłońska-Jesionowska, *Lidia Zawadzka-Głos
Diagnostic evaluation of congenital respiratory stridor in children
Diagnostyka wrodzonego stridoru oddechowego u dzieci
Department of Paediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Wstęp. Wrodzony stridor oddechowy u dzieci wynika z patologii dróg oddechowych zlokalizowanych na różnych poziomach. Nieustannie prowadzone są dyskusje, czy każdy stridor wymaga diagnostyki oraz jaka metoda jest najskuteczniejsza w postawieniu ostatecznego rozpoznania.
Cel pracy. Celem niniejszej pracy jest odpowiedź na pytanie, jakie badanie należy wykonać w diagnostyce stridoru wrodzonego u dzieci i dlaczego oraz co jest najczęstszą przyczyną takiego stridoru.
Materiał i metody. Ocenie poddano grupę 110 dzieci diagnozowanych z powodu wrodzonego stridoru oddechowego. Oceniono stan ogólny dzieci, wykonano badania obrazowe oraz endoskopię dolnych dróg oddechowych w znieczuleniu ogólnym. U niektórych dzieci wykonano ECHO serca i rtg kontrastowe przełyku czy TK klatki piersiowej z kontrastem.
Wyniki. Analizie poddano dzieci w wieku od 3 dni do 11. roku życia. Najczęstszą przyczyną stridoru była wrodzona wiotkość krtani stwierdzona u 50% badanych, a następnie anomalie wielkich naczyń tętniczych u 28% dzieci. Najczęściej diagnozowano stridor wdechowo-wydechowy, który może świadczyć o mnogich patologiach dróg oddechowych. Tylko endoskopia dolnych dróg oddechowych w znieczuleniu ogólnym pozwoliła na postawienie właściwego rozpoznania.
Wnioski. Najbardziej wartościową metodą w diagnostyce wrodzonego stridoru oddechowego jest endoskopia dolnych dróg oddechowych w znieczuleniu ogólnym, która obejmuje zarówno krtań, jak i tchawicę ze względu na współwystępowanie wad na różnych poziomach dróg oddechowych. Również z tego powodu rodzaj stridoru nie jest jednoznaczny z poziomem lokalizacji patologii w drogach oddechowych. Należy pamiętać, że anomalie wielkich naczyń tętniczych mogą być również przyczyną wrodzonego stridoru oddechowego.
Summary
Introduction. Congenital respiratory stridor in children is the result of respiratory tract pathology located at various anatomical levels. There is an ongoing debate on whether every case of stridor requires diagnostic evaluation and which method is the most effective for determining the final diagnosis.
Aim. The aim of the present study is to answer the question which procedure should be performed to diagnose the casuse of congenital stridor in children and why, and what the most common cause of the stridor is.
Material and methods. A group of 110 children examined due to congenital respiratory stridor was analysed. The children’s general condition was assessed and imaging studies and lower respiratory tract endoscopy were performed under general anaesthesia. In some children cardiac ultrasound and contrast-enhanced radiographic examination of the oesophagus or contrast-enhanced computed tomography scan of the chest were performed.
Results. Children aged 3 days to 11 years were analysed. The most common cause of stridor was congenital laryngeal hypotonia found in 50% of children and the second most common was abnormalities of the great arteries in 28% of children. Biphasic stridor, which may be a sign of multiple respiratory abnormalities, was diagnosed most frequently. It was only lower respiratory tract endoscopy performed under general anaesthesia that made it possible to make the right diagnosis.
Conclusions. The most useful diagnostic method for congenital respiratory stridor is lower respiratory tract endoscopy under general anaesthesia, which is used to evaluate both the larynx and the trachea since defects may coexist at various respiratory levels. It is also for this reason that the type of stridor is not tantamount to the level at which the pathology is located in the respiratory tract. One needs to bear in mind that abnormalities of the great arteries may also be the cause of congenital respiratory stridor.
Introduction
Respiratory stridor is a sign of respiratory pathology. Stridor may be of various severity depending on the degree of respiratory lumen narrowing. On clinical examination, apart from an additional sound during breathing, the activation of accessory respiratory muscles and an increase in the breathing rate per minute can be observed. Under normal conditions the breathing rate is 30/min in infancy and 26/min in the second year of life. Breathing may be shallow and irregular. Stridor may be present at rest or may occur during exertion, crying or feeding. In addition, hoarseness or a changed, voiceless voice quality may occur. Chronic cough and recurrent lower respiratory tract inflammation are observed, which are the result of difficulties with discharge clearance from a narrowed respiratory tract. The latter symptoms may suggest asthma or recurrent laryngitis (1). Respiratory disorders may be accompanied by digestive tract symptoms such as choking or regurgitation.
Diagnostic evaluation should be performed in every patient with the symptoms of respiratory pathology mentioned above. In every case one should start with general assessment of the patient’s clinical status, chest auscultation, saturation measurement and chest radiography. Endoscopic methods which are the basis of diagnostic evaluation of lower respiratory tract pathology include indirect laryngoscopy, laryngofiberoscopy and direct laryngoscopy. Indirect laryngoscopy using a laryngeal mirror requires full cooperation from the patient. Laryngofiberoscopy without general anaesthesia makes it possible to examine the larynx during phonation, swallowing and breathing. It allows one to evaluate functional laryngeal disorders, but only down to the level of the vocal folds. It is not possible to view the subglottic area of the larynx using this procedure (2, 3). Direct laryngoscopy under general anaesthesia makes it possible to assess all anatomical levels of the larynx: the epiglottis, glottis, subglottic area and trachea down to its bifurcation and division into the main bronchi. Due to the use of general anaesthesia the possibility of vocal fold motility assessment is limited (3). Additional diagnostic imaging methods include laryngeal ultrasound, contrast-enhanced oesophageal radiograph, cardiac ultrasound, contrast-enhanced computed tomography and magnetic resonance imaging of the chest. Due to the need for the child to cooperate with the physician during the examination not all methods are useful in paediatric laryngology. Different methods do not make it possible to evaluate all the anatomical levels at once; it is necessary to use complementary methods.
Aim
The aim of the present study is to answer the question which procedure should be performed to diagnose the cause of congenital stridor in children and why, and what the most common cause of such stridor is.
Material and methods
A group of 110 children were assessed who were evaluated at the Department of Paediatric Otolaryngology of the Medical University of Warsaw over a period of 5 years due to congenital respiratory stridor, i.e. one which appeared not later than 4 weeks from birth. Stridor was permanent or periodic and did not subside before the child was evaluated. Children with a history of prolonged endotracheal intubation were excluded from the study due to the possibility of acquired intubation-related stenosis.
In the course of the diagnostic evaluation information on the patient’s perinatal period, need for endotracheal intubation, any recurrent respiratory infections and dysphagia manifesting as choking or regurgitation was obtained. Subsequently, the following procedures were performed: paediatric examination, chest radiograph, laryngeal ultrasound with vocal fold motility evaluation, 24-hour blood oxygen saturation monitoring and lower respiratory tract endoscopy under general anaesthesia with the assessment of the epiglottis, interarytenoid space, laryngeal vestibule and laryngeal ventricles, vocal folds, the subglottic area and the trachea down to its division into the main bronchi. Depending on the type of additional symptoms the following procedures were performed: cardiac ultrasound in order to exclude heart defects in the case of auscultatory abnormalities over the heart or an enlarged heart and contrast-enhanced oesophageal radiograph in the case of dysphagia. Contrast-enhanced computed tomography of the chest was performed in children in whom lower respiratory tract endoscopy revealed airway stenosis due to external tracheal and/or bronchial compression.
Results
The children included in the study were aged 3 days to 11 years. The median age was 3 years. Out of 110 hospitalised children 56 (51%) were below 3 months of age on admission. Among the 110 studied children there were 53 boys (48%) and 57 girls (52%).
Respiratory stridor appeared in the first minutes after birth or, more commonly, between the second and fourth week of age. The following types of stridor were distinguished: permanent, periodic, infection-induced and exertional. Permanent stridor, audible during every breath was found in 74 children (67%) and periodic stridor, occurring a few to a dozen or so times a day or even every few days was observed in 29 children (26%). Infection-induced stridor, which occurs only during infection and subsides during recovery, was found in 1 child (1%). Stridor on exertion occurred in 6 children (6%): in infants during anxiety, crying and feeding and in older children during physical activity. Depending on the respiratory phase at which stridor occurred 3 types of stridor were distinguished: inspiratory stridor, which was found in 70 children (63%), expiratory stridor observed in 37 children (34%) and biphasic stridor found in 3 children (3%).
The following aspects were evaluated in the children: apnoea with blood oxygen saturation drops, voice quality and recurrent respiratory infections. Apnoea with blood oxygen saturation drops below the normal range, i.e. below 98% was observed in 24 out of 110 children (22%), i.e. in 21 out of 55 children with permanent stridor (38%), in 2 out of 4 children with exertional stridor (50%) and in only 1 out of 28 children with periodic stridor (3%). In 86 children (78%) the saturation was normal. The Pearson’s chi-square test confirmed a statistically significant relationship between permanent stridor and saturation level below the normal range (p = 0.03216). However, no relationship was confirmed between the final diagnosis of the cause of stridor and apnoea. In 12 children (11%) abnormal voice quality was observed: voice loss in 6 children and hoarseness in 6 children. Voice abnormalities were observed in 9 out of 74 children with permanent stridor, in 2 with periodic stridor and in 1 with stridor on exertion. The Fisher’s test did not reveal any statistically significant relationship between voice quality and the type of congenital respiratory stridor (p = 0.7476). A history of recurrent respiratory infections was found in 35 out of 110 children with congenital respiratory stridor (32%). Recurrent infections were defined as those occurring once a month in neonates and children aged up to 3 months and more than 6 times a year in older children. Recurrent lower respiratory tract infections were found in 14 out of 31 children (45%) with abnormalities of the great arteries and in 21 out of 79 children (27%) with other airway abnormalities causing stridor. Recurrent lower respiratory tract infections are relatively common in children with abnormalities of the great arteries; however, no statistically significant relationship was demonstrated in the Pearson’s chi-square test (p = 0.0713) between the prevalence of recurrent lower respiratory tract inflammation in children with abnormalities of the great arteries and the prevalence of such infections in children with other lower respiratory tract pathologies causing stridor.
Plain radiographs of the chest were taken for all 110 children in which the following aspects were evaluated: anatomical defects of the chest cavity, cardiac size, silhouette and position in the mediastinum and the presence of inflammatory lesions. In 98 children (86%) no abnormalities were found on chest radiograph. In 4 children atelectasis was found and in 2 children emphysema was diagnosed (6 children in total, 5%). The Fisher’s test revealed a statistically significant relationship between radiographic abnormalities and the type of stridor (p = 0.002977). These abnormalities occurred only in patents with exertional and infection-induced stridor and the probability of the presence of such abnormalities is 0.04 times higher in such patients than in those with permanent and periodic stridor.
In 46 out of 110 children with congenital respiratory stridor (42%) various forms of dysphagia were found: in 19 children (17%) choking was observed, in 12 (11%) regurgitation was found and in 15 (14%) both choking and regurgitation were observed. Gastroesophageal reflux was found in 10 children with regurgitation only using a pH test or abdominal ultrasound. The 46 children with dysphagia underwent radiographic examination of the oesophagus with barium contrast. No pathology was found in 29 out of these 46 children (63%): the contrast material passed to the stomach freely. In the remaining 17 children (37%) a loss of oesophageal shadow from the back or side was observed. Based on this, abnormalities of the great arteries compressing the oesophagus were suspected in 15% of all 110 children with congenital respiratory stridor. Based on the Fisher’s test a statistically significant relationship was found between abnormalities of the great arteries and choking (p = 0.0006748) and between abnormalities of the great arteries and regurgitation (p = 0.005389). The chance of having abnormalities of the great arteries is more than 4 times higher in children with stridor and choking (odds ratio of 4.8660020) and over 6 times larger in children with stridor and regurgitation (odds ratio of 6.696638).
All 110 children underwent laryngeal ultrasound with vocal fold motility assessment. In 95 children (86%) a normal image of the larynx was found. In 9 children (8%) restricted vocal fold motility was found and a preliminary diagnosis of vocal fold paralysis was made; in 2 children (2%) laryngeal haemangioma was diagnosed and in 3 children (3%) laryngeal cysts were found.
Cardiac ultrasound was performed in 56 children with congenital respiratory stridor and auscultatory changes in the form of heart murmur. In 21 out of 56 children (38%) the following intracardiac defects were found: ventricular septal defect (VSD), atrial septal defect (ASD), patent foramen ovale (PFO), patent ductus arteriosus (PDA) and coarctation of the aorta. In 13 out of 56 children (23%) abnormalities of the great arteries were suspected; this accounts for 12% of the whole group of 110 children with congenital respiratory stridor.
It was not possible to diagnose the cause of congenital respiratory stridor in all of the 110 children using the diagnostic procedures presented above. Based on laryngeal ultrasound the following preliminary diagnoses were made in 14 children: vocal fold paralysis in 9, laryngeal cysts in 3 and laryngeal haemangiomas in 2 children. Based on radiographic examination of the oesophagus with barium contrast abnormalities of the great arteries were suspected in 17 children and the condition was suspected in 13 children based on cardiac ultrasound. These were the same children in whom changes were discovered on oesophagus radiographs with contrast. In the remaining 79 children (72%) interview, clinical examination and additional diagnostic studies mentioned above did not provide the final diagnosis. The diagnostic methods mentioned above did not allow one to diagnose the most common causes of congenital respiratory stridor which are laryngeal hypotonia and congenital cricoid cartilage stenosis.
In all 110 children lower respiratory tact endoscopy was performed under general anaesthesia during which the final diagnosis was determined in 79 children (72%) and preliminary diagnosis was made in 31 children (28%). In 55 children (50%) congenital laryngeal hypotonia was found, in 6 (6%) vocal fold paralysis, in 5 (4%) congenital cricoid cartilage stenosis, in 5 (4%) laryngeal cysts, in 4 (4%) haemangiomas, in 3 (3%) laryngeal web and in 1 (1%) laryngeal papillomas was found. In 31 children (28%) a preliminary diagnosis was made of congenital abnormalities of the great arteries, which was subsequently confirmed on chest computed tomography (fig. 1).
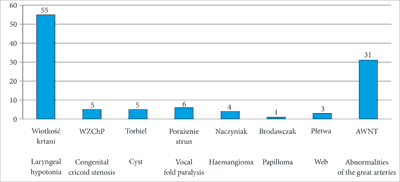
Fig. 1. Causes of congenital respiratory stridor in 110 children
In addition, in 21 children (20%) simultaneous treatment was started and in 4 tracheotomy was performed in order to secure the airway as preparation for further treatment. In 17 children (15%) complete cure was achieved by aryepiglottic fold incision, cyst marsupialisation or laryngeal web excision, which resulted in complete disappearance of respiratory stridor.
Endoscopic examination of the lower respiratory tract revealed multiple respiratory tract abnormalities in 21 children (tab. 1). In the study group 89 children (81%) had 1 lower respiratory tract pathology and 16 (14%) had 2 abnormalities; in 5 children (5%) 3 concomitant congenital airway abnormalities were found. The most common concomitant abnormalities included: congenital laryngeal hypotonia with congenital cricoid cartilage stenosis in 5 cases, laryngeal hypotonia with vocal fold paralysis in 2 cases and congenital cricoid cartilage stenosis with grade 1 laryngeal cleft in 2 cases; in a child with 3 airway pathologies the following were discovered: laryngeal hypotonia, cricoid cartilage stenosis and grade 1 laryngeal cleft.
Tab. 1. Concomitant lower respiratory tract abnormalities in the study group (n = 110)
| Number of abnormalities | Number of patients | Proportion |
| 3 | 5 | 5 |
| 2 | 16 | 14 |
| 1 | 89 | 81 |
Statistical analysis was performed of the relationship between the type of respiratory stridor associated with an anatomical level of lower respiratory tract pathology and the final diagnosis of the cause of stridor (tab. 2). In the study group of 110 children with congenital respiratory stridor the most common type of stridor was biphasic stridor, which was diagnosed in 70 children (66%). The Fisher’s test did not demonstrate any statistically significant association between this type of stridor and the anatomical level of pathology in the lower respiratory tract (p = 0.5992).
Tab. 2. Type of respiratory stridor associated with an anatomical level of pathology in the lower respiratory tract versus the final diagnosis of the cause of stridor with statistical analysis in a group of 110 children
| | Biphasic | Inspiratory | Expiratory |
| Congenital laryngeal hypotonia | 34 | 21 | 0 |
| Cricoid cartilage stenosis | 5 | 0 | 0 |
| Cyst | 1 | 3 | 1 |
| Abnormalities of the great arteries | 22 | 7 | 2 |
| Vocal fold paralysis | 1 | 5 | 0 |
| Web | 3 | 0 | 0 |
| Haemangiomas | 3 | 1 | 0 |
| Papilloma | 1 | 0 | 0 |
Discussion
The problem of respiratory stridor, which is widespread in the paediatric population, is still a matter of significant doubt regarding the need for diagnosis and treatment. The principles of the management of children with acute respiratory stridor associated with airway infection are clear. It is obvious that treatment needs to be started immediately in order to eliminate dyspnoea, which manifests as stridor, among other symptoms. If dyspnoea persists despite appropriate conservatory treatment, there is also no doubt that diagnostic evaluation is necessary in order to assess the respiratory lumen and determine the cause of dyspnoea. The situation is different in the case of congenital respiratory stridor in children.
The most common cause of congenital respiratory stridor in children is laryngeal hypotonia, or laryngomalacia. Stridor associated with laryngeal hypotonia resolves spontaneously in the majority of cases, i.e. it does not require treatment. This occurs between 18 and 24 months of age (2-5). Based on on the literature data provided above, the fact that 35-75% of congenital stridor is caused by laryngeal hypotonia which resolves spontaneously begs the logical question: which children with stridor should be medically evaluated?
In all 110 children with congenital respiratory stridor evaluated at the Department of Paediatric Otolaryngology of the Medical University of Warsaw over the period of 5 years the cause of lower respiratory tract stenosis was determined. In 55 children (50%) congenital laryngeal hypotonia was found, in 6 (6%) vocal fold paralysis, in 5 (4%) congenital cricoid cartilage stenosis, in 5 (4%) laryngeal cysts, in 4 (4%) haemangiomas, in 3 (3%) laryngeal web and in 1 child (1%) laryngeal papillomas were found. In as many as 31 children (28%) congenital abnormalities of the great arteries were diagnosed.
Congenital laryngeal hypotonia was diagnosed in 50% of children in the study. The authors of other studies also report that the most common cause of congenital stridor is laryngomalacia, with a frequency of 35-75% which is corroborated in the material from the present study (6). According to medical literature, the next lower airway abnormalities in the order of frequency which are the cause of congenital stridor include: vocal fold paralysis and congenital cricoid cartilage stenosis (6, 7). This is not corroborated by the material from this study since the second most common pathology observed in it is abnormalities of the great arteries, which were found in 31 out of 110 children (28%). Vocal fold paralysis being diagnosed in 6 children (6%) and congenital cricoid cartilage stenosis being found in 5 out of 110 children (4%) are further on the list. The authors of other studies report that abnormalities of the great arteries are very rare. They account for 0.1% of all congenital heart defects (6-8). No data have been found in the literature on how often abnormalities of the great arteries are the cause of congenital respiratory stridor.
The present study analysed the type of stridor associated with an anatomical level of airway constriction. The types of stridor include inspiratory, expiratory and biphasic stridor. Many authors report that the type of stridor may be associated with a specific anatomical level at which respiratory pathology is present (4, 9). In the present study inspiratory stridor was observed in 37 out of 110 children (34%), expiratory stridor in 3 out of 110 children (3%) and biphasic stridor in as many as 70 out of 110 children (63%). The Fisher’s test did not confirm any statistically significant association between the type of stridor and the location of pathology in the lower respiratory tract (p = 0.5992). Inspiratory stridor characteristic for abnormalities at the upper level of the larynx, which was observed in 37 children in total, was most commonly found in children with congenital laryngeal hypotonia, i.e. in 21 out of 55 children with this diagnosis (38%), but as many as 34 out of those 55 children (62%) presented with biphasic stridor. Congenital laryngeal hypotonia is an abnormality of the upper level of the larynx and should be characterised by typically inspiratory stridor. In the present study, in children with laryngeal hypotonia biphasic stridor is more common than inspiratory stridor, at a ratio of 1.6:1, which may indicate a coexistence of congenital defects at other levels of the lower respiratory tract. In 5 children with laryngomalacia and biphasic stridor additional defects were found in the subglottic area in the form of congenital cricoid cartilage stenosis. Abnormalities of the great arteries cause respiratory lumen stenosis at the level of the trachea and bronchial opening. They should be characterised by biphasic or expiratory stridor. The analysis of the present study demonstrated that all types of stridor were present in children with abnormalities of the great arteries: inspiratory in 7 (22%), expiratory in 2 (7%) and the most common biphasic one in 22 children (71%). The presence of stridor other than biphasic and expiratory is also due to multiple laryngeal and tracheal pathologies, which were found in 11 out of 31 children (36%). The authors of the quoted sources report that in nearly 50% of children with chronic stridor there are concomitant multiple lower respiratory tract abnormalities. This is equally applicable to children with laryngomalacia, congenital cricoid cartilage stenosis and congenital vocal fold paralysis (3, 5, 8). An important conclusion based on the above observations is that endoscopic examination should not be limited to selected portions of the lower respiratory tract, e.g. to the larynx only, but should cover the whole area, i.e. the larynx, subglottic area and trachea with its branching into the main bronchi, since respiratory tract defects are often multiple and may be located at different anatomical levels. In this way, the endoscopy technician is certain not miss any pathology during the diagnostic evaluation.
With the use of the abovementioned diagnostic procedures, i.e. chest radiograph, laryngeal ultrasound with vocal fold motility assessment, oesophagus radiograph with barium contrast and cardiac ultrasound performed in 110 children with congenital respiratory stridor, preliminary diagnosis was made only in 31 children (28%). In another 14 children the following preliminary diagnoses were made based on laryngeal ultrasound: restricted vocal fold motility in 9 children, laryngeal cysts in 3 and laryngeal haemangiomas in 2 children. It was only endoscopic examination of the lower respiratory tract performed in all 110 children that made it possible to make a definitive diagnosis of the cause of respiratory stridor in all children (100%). Abnormalities which constrict the airway are located at various anatomical levels: not only in the larynx, but also in the trachea. The diagnosis of pathology at the upper level of the larynx does not exclude the possibility of abnormalities in the subglottic area and in the trachea. Even when vascular abnormalities where diagnosed on oesophagus radiograph with contrast, it was endoscopy that revealed the coexistence of pathologies at the level of the vocal folds: vocal fold paralysis and laryngeal web, which were mainly responsible for the clinical symptoms and required immediate treatment. As Nicolai and other authors report (10, 11), the most common indication for lower airway endoscopy in children is congenital stridor and recurrent laryngitis. The study by Nicolai on 53 children demonstrated the presence of pathology in 96% of cases. Even the most common cause of stridor, laryngomalacia, coexists in as many as 15% of children with airway stenosis in the subglottic area and trachea in the form of congenital cricoid cartilage stenosis, haemangiomas and abnormalities of the great arteries (5, 8, 11-16). Endoscopy is essential to exclude concomitant respiratory pathologies. For this reason, laryngofiberoscopy performed after the administration of a local anaesthetic to the nasal mucosa cannot be the only endoscopic examination used for the diagnosis of congenital respiratory stridor.
Conclusions
1. Diagnostic evaluation of congenital respiratory stridor must cover not only the larynx, but also the trachea with its branching into the main bronchi primarily due to the coexistence of defects at various anatomical levels of the respiratory tract or the presence of abnormalities of the main arteries causing external tracheal and/or bronchial compression.
2. The type of stridor: inspiratory, biphasic or expiratory is not exclusively associated with a single anatomical level of respiratory tract pathology due to the coexistence of defects.
3. The most useful method for the diagnosis of the cause of congenital respiratory stridor is lower respiratory tract endoscopy using a rigid endoscope under general anaesthesia.
4. Abnormalities of the great arteries are a more common cause of congenital respiratory stridor than previously thought.
Piśmiennictwo
1. Spencer S, Yeoh BH, Aspersen PPV, Fitzgerald DA: Biphasic stridor in infancy. Med J Aust 2004; 180(7): 347-349.
2. Zawadzka-Głos L, Zajac B, Chmielik M: Laryngeal stridor: structural differences and clinical examination of the child’s larynx. New Med 2004; 4: 102-104.
3. Gryczyńska D: Otorynolaryngologia dziecięca. Alfa-Medica Press, Bielsko-Biała 2007.
4. Chmielik M: Otorynolaryngologia dziecięca. Skrypt Akademii Medycznej, Warszawa 2006.
5. Garritano FG, Carr MM: Characteristic of patients undergoing supraglottoplasty for laryngomalacia. Int J Pediatr Otorhinolaryngol 2014; 78(7): 1095-1100.
6. Bhimij S: Vascular rings clinical presentation. Medscape updated Mar 27, 2015.
7. Bovè T, Demanet H, Casimir G et al.: Tracheobronchial compression of vascular origin. Review of experience in infants and children. J Cardiovasc Surg (Torino) 2001; 42(45): 663-666.
8. Pumberger W, Voitl P, Göpfrich H: Recurrent respiratory tract infections and dysphagia in a child with an aortic vascular ring. South Med J 2002; 95(2): 265-268.
9. Swami N, Koshy G, Jamal M et al.: Typical asthmatic presentation of congenital vascular ring can masquerade a general physician. Case Rep Vasc Med 2013; 2013: 934790.
10. Nicolai T: Pediatric bronchoscopy. Pediatr Pulmonol 2001; 31(2): 150-164.
11. Murphy TD, Bye MR: Congenital stridor. Medscape: Jan 21, 2015.
12. Woods RK, Sharp RJ, Holcomb GW 3rd et al.: Vascular anomalies and compression: single institution’s 25-year experience. Ann Thorac Surgery 2001; 72: 434-439.
13. Jabłońska-Jesionowska M, Zawadzka-Głos L: Respiratory stridor in children due to congenital abnormalities of the great vessels. New Med 2015; 4: 3-5.
14. Adamczuk D, Krzemień G, Szmigielska A et al.: Wrodzony stridor krtaniowy – problem interdyscyplinarny. Developmental Period Medicine 2013; XVII(2): 174-178.
15. Hernanz-Schulman M: Vascular rings: a practical approach to imaging diagnosis. Pediatr Radiol 2005; 35(10): 961-979.
16. Lai S-H, Liao S-L, Wong K-S: Cardiovascular-associated tracheobronchial obstruction in children. Cardiol Young 2013; 23: 233-238.
