Urszula Kaczmarek1, Teresa Jackowska2, Maria Mielnik-Błaszczak3, Anna Jurczak4, *Dorota Olczak-Kowalczyk5
Individualised caries prevention with fluoride in children and adolescents – recommendations of Polish experts
Indywidualna profilaktyka fluorkowa u dzieci i młodzieży – rekomendacje polskich ekspertów
Streszczenie
Obok właściwej diety profilaktyka fluorkowa jest podstawową i skuteczną metodą zapobiegania próchnicy zębów mlecznych i stałych. Warunkiem zapewnienia jej bezpieczeństwa i skuteczności jest znajomość i przestrzeganie aktualnych zasad stosowania różnych metod i środków zawierających związki fluoru.
W ramach działalności Grupy Roboczej ds. Profilaktyki Fluorkowej Polskiego Oddziału Sojuszu dla Przyszłości Wolnej od Próchnicy (ACFF) powołano zespół ekspertów w dziedzinie stomatologii dziecięcej i pediatrii w celu aktualizacji stanowiska dotyczącego indywidualnej profilaktyki fluorkowej u dzieci i młodzieży w Polsce.
Dokonano przeglądu piśmiennictwa dotyczącego poziomu wiedzy na temat profilaktyki fluorkowej, korzystania z niej przez osoby w wieku rozwojowym w Polsce, skuteczności i bezpieczeństwa stosowania środków profilaktycznych zawierających związki fluoru oraz zaleceń w zakresie profilaktyki fluorkowej organizacji i towarzystw naukowych w różnych krajach na świecie. Pierwsza wersja dokumentu była dyskutowana i zaakceptowana przez zespół ekspertów w dziedzinie pediatrii i stomatologii dziecięcej w dniu 4 kwietnia 2019 roku. Aktualizację zaplanowano nie później niż po 5 latach od jego publikacji.
Dokument zawiera podstawowe informacje dotyczące poziomu wiedzy o profilaktyce fluorkowej rodziców, dzieci i młodzieży, mechanizmu przeciwpróchnicowego działania fluoru, bezpieczeństwa i skuteczności różnych metod indywidualnej profilaktyki fluorkowej i zasad jej stosowania w zależności od wieku i poziomu ryzyka próchnicy.
Summary
In addition to proper diet, the use of fluoride is the primary and effective method for the prevention of dental caries in primary and permanent dentition. Knowledge and compliance with the current guidelines for the use of different strategies and agents containing fluoride compounds is crucial for ensuring safety and efficacy of prevention.
A panel of experts in paediatric dentistry and paediatrics was established as part of the working group of the Polish Branch of Alliance for a Cavity-Free Future (ACFF) to update the position on individual fluoride prevention in children and adolescents in Poland.
We conducted a literature review on the knowledge of fluoride prevention, its use in the paediatric population in Poland, efficacy and safety of fluoride-containing preventive agents, as well as recommendations on fluoride prophylaxis issued by academic organisations and societies in different countries worldwide. The first version of the document was discussed and accepted by the panel of experts on paediatrics and paediatric dentistry on the 4th of April 2019. Update was scheduled for not later than 5 years after publication.
This document includes basic data on the knowledge of fluoride prevention in parents, children and adolescents, the anticariogenic mechanism of fluoride, the safety and efficacy of different methods for individual fluoride prophylaxis, and the principles for its use depending on age and the risk of caries.

Introduction
Proper nutrition, oral hygiene and prophylactic agents containing fluorides are the key elements in the prevention of carious disease. Fluorides are used in mass, group and individual prevention. The World Health Organisation and the World Dental Federation recommend their preventive use, emphasising their efficacy and safety. At the same time, they point to the need to monitor the exposure and to assess their efficacy (1-3).
Individual fluoride prevention may take the following forms:
– oral (tablets or drops) – an endogenous method (supplementation) to improve mineralisation of the developing hard dental tissues,
– external – an exogenous method involving topical application on erupted teeth.
Toothpastes, mouthwashes, foams, gels and varnishes contain different levels of fluoride. They can be used either in a home setting (home prevention) or in a dental office (professional prevention). Caries prevention with fluoride is safe and effective only when the principles of individual preventive method selection are followed, considering exposure to fluoride from other sources, child’s age and the risk of caries. According to a survey conducted in 2014 in Poland, 14.0% of dentists do not estimate the risk of caries before selecting preventive strategy. More than a half of respondents believed that fluoride prevention should be used in all patients, 38.9% claimed that it should be used at a high risk of caries and irrespective of age, and 21.3% responded that fluoride should be used only for primary and mixed dentition (4).
It should be emphasised that the basic caries prevention in Poland may be provided as part of public health service (List of general dental benefits for children and adolescents under the age of 18 years as well as List of dental benefits for children older than 6 months of age up to the age of 19 years in the form of 13 age-tailored packages of preventive treatments).
In 2013 and 2015, statements of the Independent Expert Panel on individual caries prevention were published (5, 6). Due to the disseminated false information about harmful effects of fluoride prophylaxis and, at the same time, new studies on the safety and efficacy of fluoride-containing preventive agents, an update of this document is needed.
Methodology
The working group of The Alliance for a Cavity-Free Future (ACFF) conducted a review of current literature on dental preventive needs of children and adolescents in Poland; the mechanisms of action, efficacy and safety of fluoride compounds in caries prevention in this group of patients, as well as an analysis of recommendations for caries prevention developed by academic societies, such as: American Academy of Paediatric Dentistry (AAPD), European Academy of Paediatric Dentistry (EAPD), American Dental Association (ADA), and World Dental Federation (FDI) (1, 2, 7-9). The obtained data were the basis for the document discussed and approved on the 4th April 2019 by a panel of experts composed of the representatives of the Polish Society of Paediatric Dentistry, Section of Paediatric Dentistry of the Polish Dental Society, and national consultants in the field of paediatric dentistry and paediatrics. The document will be updated not later than 5 years after publication.
Results
Dental caries prevention needs in Polish children
The need for dental caries prevention among Polish children has been confirmed by epidemiological studies conducted since 1987 in collaboration with the World Health Organisation (6-9). Current monitoring studies indicate a 2.5-fold (1.85 to 4.66) increase in the mean number of carious teeth and about 40%-increase (41.1 to 81.9%) in the incidence of caries in children aged 3-6 years. Caries affects about 3.75 teeth in 12-year-olds, and this number increases by 1.08 after 3 years (4.88 at the age of 15 years), and by 1.62 after further 3 years (reaching 6.50 at the age of 18 years) (10-12). The presence of caries in the erupting first permanent molars is alarming: it is found in 8 5-year-olds, 19 6-year-olds and 59 7-year-olds per 100 children examined. When considering the trends in carious disease among 12-year-olds within 27 years (1987-2016), a slight decrease in the frequency (from 89.9 to 85.4%) and severity (4.4 to 3.75) of caries, with significant fluctuations in this period) was observed (10-12).
Recent epidemiological studies have also shown that fluoride prophylaxis is underused among Polish children, it is introduced late and that parental knowledge on the principles of its use is insufficient (10-13).
In 2017, only one in two 3-year-olds had their teeth brushed at least twice daily, 36.4% had their teeth brushed once daily, and 8.8% – only 1-3 times weekly. Unfortunately, one in five parents had no knowledge on whether their child’s toothpaste contained fluoride, and 27.5% parents admitted that they used fluoride-free toothpaste. Excessive amount of toothpaste was used in one in three 3-year-olds and only one in three children had their teeth brushed by an adult. Fluoride varnish was used in only 10.7% of children (11). Similarly, many adolescents are uncertain whether their toothpaste contains fluoride. The lack of this knowledge was reported for 43.0% of 12-year-olds (2016), 44.7% of 15-year-olds (2018) and 52.1% of 18-year-olds (2017) (10-12). Nearly half of 12- and 18-year-olds were unaware that the use of fluoride-containing preventive agents helps prevent dental caries and that there are fluoride-containing agents other than toothpastes (10, 12).
Long-term observational studies showed that the level of caries is related to fluoride levels in drinking water. It is estimated, based on more than 100 studies conducted in 23 countries before 1990 that caries reduction due to water fluoridation is 40-50% for primary teeth and 50-60% for permanent teeth. A review of studies conducted between 1990 and 2000 indicates caries reduction of 30-59% for primary teeth and 40-49% for permanent teeth (3). Optimal fluoride level in drinking water for dental protection is estimated at about 0.7 mg/L (0.5-1 mg/L) (3). According to the World Health Organisation, the level should not exceed 1.5 mg F/L. In many regions of the world with trace or low fluoride levels in water, optimal cariostatic levels of fluoride compounds are added to water. It was demonstrated that water fluoridation is an effective and safe method for the prevention of dental caries in both children and adolescents. It was also found that the benefits of water fluoridation for the reduction of caries significantly outweigh potential adverse aesthetic effects, i.e. very mild to mild dental fluorosis. Salt or milk fluoridation is practiced in many regions of the world (e.g. Germany, Switzerland, France, Latin America) (2, 3).
In Europe, artificially fluoridated water is used by 71% of Irish people, 10% of people in Great Britain and Spain, and 3% of people in Serbia, 75% of Americans, 70% of Australians, about 50% of people in New Zealand and 38.7% of Canadians (14).
The majority of people in Poland use water with fluoride content below 0.5 mg/L, i.e. the lower range value recommended by the WHO (1994). Data on fluoride content in drinking water, which were collected based on the current literature, are shown in figure 1 (15-17).
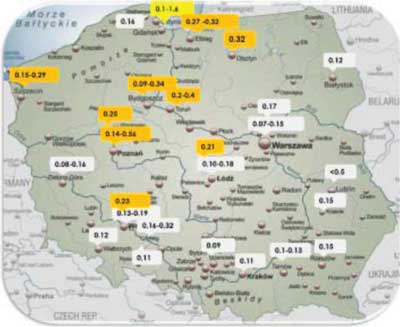
Fig. 1. Fluoride levels in drinking water in selected Polish cities based on current literature (15-17)
The cited epidemiological data and data on the exposure of Polish society to fluoride in drinking water emphasise the need for enhanced preventive and educational measures focused on the safety, principles and efficacy of fluoride prophylaxis among parents, children and adolescents in Poland. It is also important to involve not only dental personnel, but also medical professionals, class tutors and teachers in these activities. A comparative analysis of questionnaire studies among primary teachers in the years 2008 and 2018 showed a lower proportion of respondents who considered fluoride prophylaxis as beneficial (74.5 to 53.6%) (12).
Epidemiological studies have further shown polarisation of dental caries, i.e. the presence of persons with high dental caries rates even when the mean rates are generally low. It therefore seems reasonable to identify high-risk individuals and implement intensive, individualised prevention in this group of patients.
The risk of caries and fluoride prevention
The choice of preventive strategy and the type of fluoride products depends on multiple factors, including age, general health condition, preventive and therapeutic needs, caries risk level, exposure to fluoride from other sources, as well as parental engagement and possibilities. Caries prevention based on the assessment of caries risk level involves the intensification of prevention when the risk increases. Risk estimation is needed before developing an individualised preventive plan.
The risk of caries is understood as the likelihood of new carious lesions and progression of already existing ones in the future. According to the concept of dynamic balance between demineralisation and remineralisation, caries risk estimation is based on the relationship between factors considered to be preventive, i.e. which promote remineralisation (use of fluoride, antibacterial agents and fissure sealants, proper dietary habits, normal salivary flow) and caries indicators (presence of incipient caries in the form of white spots, enamel defects promoting plaque retention, number of fillings placed before 3 years of age) and risk factors for dental caries (the presence of cariogenic bacteria, reduced salivary flow, improper dietary habits), which promote demineralisation (18-20).
There are several tools for caries risk assessment. The American Academy of Pediatric Dentistry (AAPD) proposed the CRA system (Caries Risk Assessment), which consists of three tools for caries risk evaluation, including two to be used by dental practitioners: for children aged 0-5 and > 6 years, and one to be used by non-dental medical professionals for children aged 0-3 years (20). CRA is easily applicable in the clinical practice and enables caries risk evaluation (low, moderate or high). However, it requires a community interview, general medical and dental examination and is largely based on the doctor’s knowledge and clinical experience. The use of risk assessment systems additionally increases the awareness of patients and their parents/legal guardians of the causes of carious disease and helps develop medical recommendations.
Cariostatic mechanisms of fluoride
There is ample evidence confirming that fluoride compounds are effective for caries prevention and non-invasive treatment of incipient caries.
Endogenous oral administration of an optimal fluoride dose during tooth development increases fluoride content in the superficial enamel layer, allowing for a stable apatite crystalline structure to be formed. Fluoride has an impact on primary mineralisation of the organic matrix and pre-eruptive enamel maturation. It catalyses a reaction that produces hydroxyapatite, Ca10(PO4)6(OH)2. By replacing hydroxyl ions (OH-), it forms fluoridated hydroxyapatite, Ca5(PO4)3OH1-xFx. It promotes the formation of larger apatite crystals with lower carbonate content. Fluoride is also involved in pre-eruptive enamel maturation, which involves water and protein removal from the primary enamel (21).
Until recently, it was believed that lower susceptibility of enamel to acids is an effect of pre-eruptive fluoride action. However, fluoride content of enamel has no significant effects on the risk of caries. Furthermore, its excess intake may lead to dental fluorosis (3, 21-23). It therefore seems that exogenous effects of fluoride, which ensure systematic delivery of small amounts of fluoride into the oral cavity after tooth eruption, are more important.
The post-eruptive anticariogenic action of fluoride involves:
1. Limiting the effects of cariogenic bacteria by:
– reduced acid production,
– reduced plaque deposition on tooth surfaces (by disturbing the synthesis of extracellular bacterial polysaccharides),
– inhibiting bacterial carbohydrate metabolism (e.g. by reducing enolase activity, impairing glucose transport into cells, and impairing the formation of intracellular storage polysaccharides).
2. Supporting remineralisation (fluoride ions attract calcium and phosphates – new dental mineral is formed; the presence of very low F levels (> 0.03 ppm) in the dental environment, which are maintained (at 0.03-0.10 ppm) for several hours after the use of fluoridated toothpaste, are sufficient for increased remineralisation) and inhibiting demineralisation (formation of fluorapatite/ fluorohydroxyapatite which is more resistant to acid damage).
Teeth are composed of hydroxyapatite and carbonate apatite, which shows higher solubility in acids. Partially demineralised carbonate apatite crystals become nucleators to which fluoride ions are absorbed, attracting calcium and phosphate ions. As a result, a fluorapatite-like coating (without carbonate ions) is formed, making the crystal more resistant to dissolution in acids.
After application of products with fluoride content < 50 ppm with acidic or neutral pH (slower formation), fluorohydroxyapatite is formed. Preparations with fluoride content > 100 ppm at acidic pH or > 300 ppm F at neutral pH ensure formation of calcium fluoride (formed with calcium from previously dissolved enamel) which is a reservoir of fluoride ions released during bacterial acid attacks (fig. 2 and 3) (21, 24, 25).
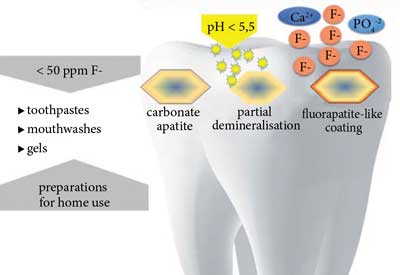
Fig. 2. Formation of more acid-resistant fluorohydroxyapatite with fluorapatite-like coating at low fluoride ion levels in the oral cavity
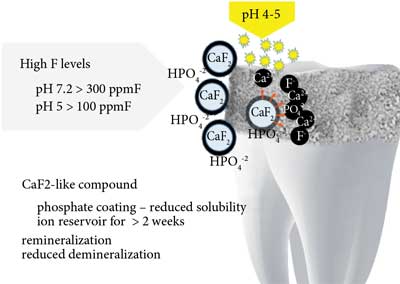
Fig. 3. Formation of calcium fluoride at high fluoride ion levels in the oral cavity
Safety of fluoride use in children
Fluoride should be used carefully and reasonably due to its high cytotoxicity and a very narrow margin between its toxic and therapeutic doses. Neglecting the principles of fluoride caries prevention is a risk factor for acute and chronic poisoning. The WHO underlined the role of fluoride exposure monitoring to assess the balance in the severity of dental caries in children at risk of dental fluorosis resulting from the accumulation of fluoride from various sources. FDI recommends the development of health policies individually for a given country and advises monitoring of the efficacy of caries prevention (1-3).
The risk of dental fluorosis depends not only on fluoride exposure, but also on individual fluoride sensitivity in a given population. That is why mild fluorosis may be observed also in areas where fluoride content in drinking water is within a range considered optimal, i.e. 0.5-1.0 mg/L (3, 22, 23, 26, 27, WHO 1994).
It is assumed that fluorosis is caused by the accumulation of fluoride doses from various sources (e.g. bottled water, tea, fish products, imported food produced in areas with fluoridated water). Excessive fluoride exposure during the so-called critical developmental period, i.e. between 15 and 30 months of age, can cause fluorosis of permanent anterior teeth and first molars, whereas other teeth may be affected at a later age (up to 6 years of age). Fluorosis may be caused by:
– preparation of infant formula using water with high fluoride concentrations (fluoride content in bottled water in Poland ranges from 0.1 to 1.39 mg F/L) (28),
– improper fluoride supplementation (other dietary components should be considered when establishing indications for this type of prophylaxis for a child) (29, 30),
– ingestion of preventive agents that are incorrectly used in children, e.g. applying too much toothpaste on a toothbrush and using products for home prevention with very high fluoride content (31).
The estimated daily intake of dietary and toothpaste fluoride (without fluoride supplementation) for 2-year-olds at fluoride content in drinking water of 1 mg/L vs. fluoride-free water is 0.069 mg F/kg body weight and 0.046 mg F/kg body weight, respectively (tab. 1) (32).
Tab. 1. Estimated daily intake of dietary and toothpaste fluoride in 2-year-olds, including fluoride supplementation (32)
| | Estimated daily intake (range) mg F/kg BW |
| Drinking water fluoride mg/L (ppm) | 1 ppm | 0 ppm |
| Diet (including water and beverages) | 0.046 (0.038-0.046) | 0.023 (0.015-0.023) |
| Toothpaste 1000 ppm F | 0.023 (0-0.154) | 0.023 (0-0.154) |
| Total | 0.069 (0.038-0.20) | 0.046 (0.015-0.177) |
| Fluoride supplementation | – | 0.038 |
| Including fluoride supplementation | 0.069 (0.038-0.20) | 0.084 (0.054-0.215) |
Fluoride content in the diets of 1-4 year olds in 16 cities located in different regions of Poland with its drinking water levels of 0.09-0.32 ppm F was estimated at 0.04 to 0.42 mg/kg (mean level 0.15 ± 0.07 mg/kg), regardless of the season. Daily fluoride intake by a child at this age was estimated at 0.28 (0.09-0.82 mg) (17). Total men fluoride intake with food and toothpaste in children will therefore not exceed the appropriate daily intake, i.e. 0.7 mg F/day for 1-3 year olds (tab. 2).
Tab. 2. Adequate intake and upper intake level depending on child’s age (33, 34)
| Age | Adequate fluoride intake (AI) in mg/day (AI) | Upper intake level of fluoride in mg/day (UL) |
| 0-6 months | 0.01 | 0.7 |
| 6-12 months | 0.5 | 0.9 |
| 1-3 years | 0.7 | 1.3 |
| 4-8 years | 1.0 | 2.2 |
| 9-13 years | 2.0 | – |
| 14-18 years | 3.0 | – |
Considering both safety and efficacy of fluoride prophylaxis, academic societies, including EAPD, AAPD, ADA and FDI, developed evidence-based standards for fluoride usage in children (1, 2, 7-9). The following aspects were considered when developing the recommendations:
– possible daily fluoride intake including its water, food and preventive agents,
– daily adequate intake (AI) of fluoride (tab. 2),
– upper intake level (UL) of fluoride that causes no observed adverse effects in the form of dental fluorosis (no-observed-adverse-effect-level).
Based on the correlations between fluoride intake and the occurrence and severity of fluorosis, it has been estimated that moderate fluorosis occurs at an intake of 0.1 mg F/kg body weight daily in less than 5% of the population (tab. 2) (33, 34).
The currently acknowledged principles of caries prevention with fluoride are to avoid excessive endogenous fluoride exposure and intensify preventive measures depending on the level of caries risk. Excessive (over-optimal) endogenous intake of fluoride in the period of the risk of fluorosis should be avoided, particularly in children below 6 years of age, by:
– limiting the amount of toothpaste with 1,000 ppm F (0.1% F) and using it in children under the age of 8 years under parental supervision, as well as using toothpastes containing 5,000 ppm F (0.5% F) from the age of 16 years as recommend by a dentist,
– introducing fluoride mouthwashes, gels and foams after the age of 6 years (fluoride varnishes can be used with no age restriction),
– restricted use of endogenous caries prevention methods (7-9, 35-37).
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. FDI: Promoting Oral Health through Water Fluoridation. Aktualizacja 2014. https://www.fdiworlddental.org/resources/policy-statements-and-resolutions/promoting-oral-health-through-water-fluoridation.
2. FDI: Promoting Dental Health through Fluoride Toothpaste. Aktualizacja 2018. https://www.fdiworlddental.org/resources/policy-statements/promoting-dental-health-through-fluoride-toothpaste.
3. O’Mullane DM, Baez RJ, Jones S et al.: Fluoride and Oral Health. Community Dental Health 2016; 33: 69-99.
4. Kaczmarek U, Majewska L, Olczak-Kowalczyk D: Postawa i wiedza stomatologów w zakresie profilaktyki fluorkowej. Nowa Stomatol 2015; 20(1): 23-28.
5. Adamowicz-Klepalska B, Borysewicz-Lewicka M, Dobrzańska A et al.: Aktualny stan wiedzy na temat indywidualnej profilaktyki fluorkowej choroby próchnicowej u dzieci i młodzieży. Niezależny Panel Ekspertów. J Stoma 2013; 66(4): 428-453.
6. Olczak-Kowalczyk D, Borysewicz-Lewicka M, Adamowicz-Klepalska B et al.: Stanowisko polskich Ekspertów dotyczące indywidualnej profilaktyki fluorkowej choroby próchnicowej u dzieci i młodzieży. Nowa Stomatol 2016; 21(1): 47-73.
7. AAPD: Fluoride Therapy. Aktualizacja 2018; https://www.aapd.org/globalassets/media/policies_guidelines/bp_fluoridetherapy.pdf.
8. EAPD: Guidelines on the use of fluoride in children: an EAPD policy document. Eur Archf Paediatr Dent 2009; 10(3): 129-135.
9. ADA Fluoridation Policy; https://www.ada.org/en/public-programs/advocating-for-the-public/fluoride-and-fluoridation/ada-fluoridation-policy.
10. Olczak-Kowalczyk D, Kaczmarek U, Bachanek T et al.: Monitorowanie stanu zdrowia jamy ustnej populacji polskiej w latach 2016-2020. Ocena stanu zdrowia jamy ustnej i jego uwarunkowań w populacji polskiej w wieku 5, 7 i 12 lat w 2016 roku. Dział Redakcji i Wydawnictw Warszawskiego Uniwersytetu Medycznego, Warszawa 2017.
11. Olczak-Kowalczyk D, Mielczarek A, Kaczmarek U et al.: Ocena stanu zdrowia jamy ustnej i jego uwarunkowań w populacji polskiej w wieku 3, 18 oraz 35-44 lata w 2017 roku. Dział Redakcji i Wydawnictw Warszawskiego Uniwersytetu Medycznego, Warszawa 2018.
12. Olczak-Kowalczyk D, Turska-Szybka A, Kaczmarek U et al.: Monitorowanie stanu zdrowia jamy ustnej populacji polskiej w latach 2016-2020. Ocena stanu zdrowia jamy ustnej i jego uwarunkowań w populacji polskiej w wieku 6, 10 i 15 lat w 2018 roku. Dział Redakcji i Wydawnictw Warszawskiego Uniwersytetu Medycznego, Warszawa 2019.
13. Turska-Szybka A, Świątkowska M, Walczak M, Olczak-Kowalczyk D: What do parents know about the use of fluoride products in children? A questionnaire study. Fluoride 2018; 51(2): 114-121.
14. https://en.wikipedia.org/wiki/Fluoridation_by_countr; https://www.canada.ca/en/services/health/publications/healthy-living/community-water-fluoridation-across-canada-2017.html.
15. Borysewicz-Lewicka M , Opydo-Szymaczek J: Fluoride in Polish drinking water and the possible risk of dental fluorosis. Pol J Environ Stud 2016; 25(1): 9-15.
16. Olczak-Kowalczyk D, Turska-Szybka A, Gozdowski D, Kaczmarek U: Defekty rozwojowe szkliwa u młodzieży w wieku 18 lat w Polsce: rozpowszechnienie i wybrane czynniki socjodemograficzne. Badania przekrojowe. Nowa Stomatol 2018; 23(2): 47-54.
17. Jędra M, Sawilska-Rautenstrauch D, Gawarska H, Starski A: Zawartość fluoru w całodziennych racjach pokarmowych małych dzieci w Polsce. Roczn PZH 2011; 62(3): 275-281.
18. Featherstone JD: The caries balance: The basis for caries management by risk assessment. Oral Health Prev Dent 2004; 2 (suppl. 1): 259-264.
19. Featherstone JD, Adair SM, Anderson MH et al.: Caries management by risk assessment: Consensus statement, April 2002. J Calif Dent Assoc 2003; 31(3): 257-269.
20. AAPD: Guideline on Caries-risk Assessment and Management for Infants, Children, and Adolescents 2014; http://www.aapd.org/media/policies_guidelines/g_cariesriskassessment.pdf.
21. Kaczmarek U: Mechanizmy kariostatyczne fluoru. Czas Stomatol 2005; 6: 404-413.
22. D’Hoore W, Van Nieuwenhuysen JP: Benefits and risks of fluoride supplementation: caries prevention versus dental fluorosis. Eur J Pediatr 1992; 152: 613-617.
23. Dąbrowska E, Balunowska M, Letko E: Zagrożenia wynikające z nadmiernej podaży fluoru. Nowa Stomat 2001; 4(18): 22-27.
24. ten Cate JM, Larsen MJ, Pearce EIF, Fejerskov O: Chemical interactions between the tooth and oral fluids. [In:] Fejerskov O, Kidd EAM (eds.): Dental caries. The disease and its clinical management. Blackwell Munksgaard, Oxford 2003; 49-70.
25. Ogaard B: CaF2 formation: cariostatic properties and factors of enhacing the effect. Caries Res 2001; 35 (suppl. 1): 40-44.
26. European Commision: Directorate-Deneral for Health & Consumers. Scientific Committee on Health and Environmental Risks SCHER: Critical review of any new evidence on the hazard profile, health effects, and human exposure to fluoride and the fluoridating agents of drinking water. SCHER 16.05.2011.
27. Public Health England: Water Fluoridation: Health monitoring report for England 2018; https://www.gov.uk/government/publications/water-fluoridation-health-monitoring-report-for-england-2018.
28. Borysewicz-Lewicka M, Chłapowska J, Wagner L, Trykowski J: Ocena zawartości fluorków w niektórych krajowych wodach mineralnych. Czas Stom 1999; 52(1): 29-32.
29. Opydo-Szymaczek J: Znaczenie oceny ekspozycji na fluorki w profilaktyce stomatologicznej. Stomat Współczesna 2003; 5(10): 44-48.
30. Opydo-Szymaczek J: Fluoride Exposure from Diet in Infants and Young Children Fed with the Foodstuffs for Particular Nutritional Uses. Dent Med Probl 2012; 49(2): 209-215.
31. Borysewicz-Lewicka M, Opydo-Szymaczek J, Opydo J: Fluoride ingestion after brushing with a gel containing a high concentration of fluoride. Biol Trace Elem Res 2007; 120(1-3): 114-120.
32. Mejáre I: Current guidance for fluoride intake: is it appropriate? Adv Dent Res 2018; 26: 167-176.
33. Dietary Reference intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride: Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine. National Academy Press, Washington, D.C. 1997; 288-313.
34. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Fluoride. The EFSA Journal 2005; 192: 1-65.
35. AAPD: Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. 2014; http://www.aapd.org/media/policies_guidelines/p_eccclassifications.pdf.
36. Weyant RJ, Tracy SL, Anselmo TT et al.; American Dental Association Council on Scientific Affairs Expert Panel on Topical Fluoride Caries Preventive Agents: Topical fluoride for caries prevention: executive summary of the updated clinical recommendations and supporting systematic review. J Am Dent Assoc 2013; 144(11): 1279-1291.
37. Rozier RG, Adair S, Graham F et al.: Evidence-Based Clinical Recommendations on the Prescription of Dietary Fluoride Supplements for Caries Prevention. A report of the American Dental Association Council on Scientific Affairs. JADA 2010; 141(12): 1480-1489.
38. Beltran-Aguilar ED, Barker LK, Canto MT et al.: Surveillance for dental caries, dental sealants, tooth retention, edentulism, and enamel fluorosis: United States, 1988-1994 and 1999-2002. MMWR Surveill Summ 2005; 54: 1-43.
39. Wright JT, Hanson N, Ristic H et al.: Fluoride toothpaste efficacy and safety in children younger than 6 years. J Am Dent Assoc 2014; 145(2): 182-189.
40. McPherson CA, Zhang G, Gilliam R et al.: An Evaluation of Neurotoxicity Following Fluoride Exposure from Gestational Through Adult Ages in Long-Evans Hooded Rats. Neurotox Res 2018; 34(4): 781-798.
41. FDI: Topical and Systemic Fluorides in Children with Renal Diseases. Aktualizacja 2009; https://www.fdiworlddental.org/resources/policy-statements-and-resolutions/topical-and-systemic-fluorides-in-children-with-renal.
42. Tubert?Jeannin S, Auclair C, Amsallem E et al.: Fluoride supplements (tablets, drops, lozenges or chewing gums) for preventing dental caries in children. Cochrane Database Syst Rev 2011; (12): CD007592.
43. Steinbacher DM, Glick M: The dental patient with asthma. An update and oral health considerations. JADA 2001; 132: 1229-1239.
44. Iida H, Kumar JV: The association between enamel fluorosis and dental caries in U.S. schoolchildren. JADA 2009; 140: 855-862.
45. Korporowicz E, Rożniatowski P, Sobiech P, Kochman K: Rodzaj i ilość past do zębów używanych przez rodziców u dzieci w wieku od 1 do 7 lat. Nowa Stomatol 2014; 3: 124-126.
46. Walsh T, Worthington HV, Glenny AM et al.: Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2010; 20(1): CD007868.
47. American Dental Association Council on Scientific Affairs: Fluoride toothpaste use for young children. J Am Dent Assoc 2014; 145(2): 190-191.
48. Al-Mulla A, Karlsson L, Kharsa S et al.: Combination of high-fluoride toothpaste and no post-brushing water rinsing on enamel demineralization using an in situ caries model with orthodontic bands. Acta Odontol Scand 2010; 68(6): 323-328.
49. Nordström A, Birkhed D: Preventive effect of a high-flu oride dentifrice (5,000 ppm) in caries-active adolescents – a 2-year clinical trial. Caries Res 2010; 44: 323-333.
50. Marinho VCC, Higgins JP, Logan S, Sheiham A: Fluoride mouthrinses for preventing dental caries in children and adolescents. Cochrane Database Syst Rev 2003; 3: CD002284.
51. Marinho VCC: Cochrane fluoride reviews: an overview of the evidence on caries prevention with fluoride treatments. RCS 2014; 5(2): 78-83.
52. Alexander SA, Ripa LW: Effects of self-applied topical fluoride preparations in orthodontic patients. Angle Orthod 2000; 70: 424-430.
53. O’Reilly MM, Featherstone JD: Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop 1987; 92: 33-40.
54. Twetman S, Keller MK: Fluoride Rinses, Gels and Foams: An Update of Controlled Clinical Trials. Caries Res 2016; 50 (suppl. 1): 38-44.
55. Sköld UM, Birkhed D, Borg E, Petersson LG: Approximal caries development in adolescents with low to moderate caries risk after different 3-year school-based supervised fluoride mouth rinsing programmes. Caries Res 2005; 39: 529-535.
56. Zero DT, Fu J, Espeland MA, Featherstone JD: Comparison of fluoride concentrations in unstimulated whole saliva following the use of a fluoride dentifrice and a fluoride rinse. J Dent Res 1988; 67: 1257-1262.
57. Duckworth RM, Horay C, Huntington E, Mehta V: Effects of flossing and rinsing with a fluoridated mouthwash after brushing with a fluoridated toothpaste on salivary fluoride clearance. Caries Res 2009; 43: 387-390.
58. Driscoll WS, Swango PA, Horowitz AM, Kingman A: Caries-preventive effects of daily and weekly fluoride mouthrinsing in a fluoridated community: final results after 30 months. J Am Dent Assoc 1982; 105: 1010-1013.
59. Heifetz SB, Meyers RJ, Kingman A: Comparison of the anticaries effectiveness of daily and weekly rinsing with sodium fluoride solutions: findings after three years. Pediatr Dent 1983; 4: 300-303.
60. Marinho VCC, Worthington HV, Walsh T, Chong LY: Fluoride gels for preventing dental caries in children and adolescents. Cochrane Clinical Answers 2015; http://cochraneclinicalanswers.com/doi/10.1002/cca.876/full.
61. Ekstrand J, Koch G, Lindgren LE, Petersson LG: Pharmacokinetics of fluoride gels in children and adults. Caries Res 1981; 15(3): 213-220.
62. Whitford GM: The metabolism and toxicity of fluoride. Monogr Oral Sci 1989; 13: 1-160.
63. Pendrys DG, Haugejorden O, Bårdsen A et al.: The risk of enamel fluorosis and caries among Norwegian children: implications for Norway and the United States. J Am Dent Assoc 2010; 141(4): 401-414.
64. Milgrom P, Taves DM, Kim AS et al.: Pharmacokinetics of fluoride in toddlers after application of 5% sodium fluoride dental varnish. Pediatrics 2014; 134(3): e870-874.
65. Browne D, Whelton H, O’Mullane D: Fluoride metabolism and fluorosis. J Dent 2005 Mar; 33(3): 177-186.
66. Holve S: An observational study of the association of fluoride varnish applied during well child visits and the prevention of early childhood caries in American Indian children. Matern Child Health J 2008; 12 (suppl. 1): 64-67.
67. Garcia RI, Gregorich SE Ramos-Gomez F et al.: Absence of Fluoride Varnish-Related Adverse Events in Caries Prevention Trials in Young Children, United States. Prev Chronic Dis 2017; 14: 160372.
68. Walczak M, Turska-Szybka A: The efficacy of fluoride varnishes containing different calcium phosphate compounds. Fluoride 2017; 50 (1 Pt 2): 151-160.
69. Turska-Szybka A, Soika I, Rozniatowski P et al.: Preventive effectiveness of fluoride varnishes in preschoolers: randomized controlled trials. FDI World Dental Congress, Madrid (29th August-1st September), Hiszpania 2017: S.212.



