© Borgis - Postępy Nauk Medycznych 2/2011, s. 109-115
*Bogusław Maciejewski
Radioterapia teragnostyczna
Theragnostic Radiotherapy
Department Radiotherapy Centre of Oncology, M. Sklodowska-Curie, Memorial Institute, branch Gliwice
Head of Department: prof. dr hab. med. Bogusław Maciejewski
Streszczenie
Praca prezentuje aktualne problemy radioterapii, nowe rozwiązania technologiczne i terapeutyczne oraz perspektywy postępu. Niektóre stare dogmaty są przedmiotem krytycznej dyskusji. W erze 3D obrazowania (TK, NMR, PET) klasyfikacja zaawansowania wolumetrycznego wydaje się konkurencyjna w stosunku do klasycznego rangowego systemu TNM. Dawka i jej frakcjonowanie wymaga indywidualnego dopasowania do wyjściowej liczby komórek nowotworowych (objętość), a nie do stopnia zaawansowania T. Fundamentalny dogmat, że takie same dawki (frakcje) skutkują śmiercią takiego samego odsetka komórek nowotworowych utracił wiarygodność. Praktyka kliniczna wskazuje, że szereg metod 3D konformalnej radioterapii ciągle nie wykazała dowodu I lub II stopnia. W profilowaniu proteomicznym i molekularnym indywidualnych guzów nowotworowych upatrywano następnego „Świętego Grala” dla radioterapii. Jednak szereg badań dowodzi, że ta obiecująca perspektywa wydaje się znacznie bardziej złożona i niejednorodna niż początkowo sądzono. W świetle dotychczasowych osiągnięć i rozczarowań „Radioterapia (Onkologia) Teragnostyczna” jest obiecująca. To pojęcie oznacza wykorzystanie wiedzy i doświadczenia multidyscyplinarnego zespołu narządowego w celu zaplanowania zindywidualizowanej kombinacji metod terapeutycznych, ich sekwencji i ścisłego reżimu czasowego.
Summary
Present paper presents actual controversial problems of radiotherapy, new technological and therapeutic developments and future perspectives. Some old dogmas are critically discussed. In the era of 3D imaging (CT, MRI, PET) volumetric staging should be used rather than classic rank TNM. Optimal and individual dose and fractionation should be taylored to the initial tumour cell number (volume) but not to T stage. Fundamental dogma that equal doses (fractions) kill the some rate of cancer cells is not longer valid. Biological concept and practical consequence of “hypofractionation back to bedside” is presented. Clinical practice shows that many of 3D conformal RT methods still do not reach level I or II evidence. Proteomic and molecular tumour profiling have been advocated as the next “Holly Grail” for radiotherapy. However many studies show that this promising issue seems to be more complex and heterogeneous as it has been assumed previously. In the light of the present achievements and disappointments, “Theragnostic Radiotherapy” (oncology) provides some promising perspective. This term means to explore knowledge and experience by tumour-type oriented multidisciplinary team of oncologists to plan individually personalized combination of treatment methods, its sequence and restricted timing.

This is difficult to cover a large field of many different initiatives and innovations leading to progress in oncology and specifically radiation therapy and to present only major achievements in this field. Therefore, we deliberately resign to review the results of translation studies and clinical trials and we focus on some dogmas, new concepts and developments, doubts and uncertainties.
Dogmas die slowly
Since Fletcher defined radiobiological basis for fractionated radiotherapy that equal doses of radiation (also chemotherapy agents) kill the same rate (not a number) of cancer clonogenic cells is unalterably accepted in the practice although it is no longer true. Time factor was misprized for a long time until late eighties. It was an obvious and groundless dogma that solid tumours generally grow slowly and therefore there is no need to complete the treatment in less than 6-7 weeks. Well documented phenomenon of accelerated repopulation of clonogenic cancer cells became an important factor for treatment outcome. Any extension of treatment time results in significant decrease in local tumour control (fig. 1), including duration of chemotherapy and any time intervals between various treatment modalities used in combined therapy. Individual tumour cel characteristics are no longer considered as homogenous. The size and localization within the tumour burden of the subpopulations of clonogenic, quiescent, hypoxic, potentially apoptotic and intrinsically resistant cells are highly heterogenous and they need heteregenous dose distribution (including chemotherapy agents). This dogma should die but it does not.
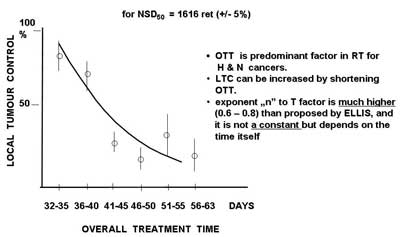
Fig. 1. Local tumour control (Maciejewski and Trott, 1983) as a function of overall treatment time for 310 T3-4N0 laryngeal cancers irradiated with a total dose for 50% local tumour control (TCD50) equivalent to Nominal Standard Dose NSD50 = 1616 reto (? 5%). For constant dose, extension of overall treatment time results in significant decrease of local tumour control.
The next even more important dogma relates to the use of the TNM staging (FIGO, UICC a bit later) as a fundamental criteria for qualification to therapy irrespectively what treatment modality is selected. In the former time, the TNM staging was based on clinical examination and very simple 2D radiologic imaging. For decades technologic revolution has offered many exquisite radiologic devices, e.g. CT, MRI, MRI Spect, US, PET, CBCT which provide functional and quantitative volumetric imaging of the primary and/or metastatic tumour(s). Moreover, radiotherapy has changed from 2D to 3D (or even 4D) including palliation but the dogmatic TNM staging is still used for treatment planning. This seems to be unexplicable nonsense. For example conventional 70 Gy in 35 fraction given to T2N0M0 oral cavity sq.c.c. might be enough for 2.1 cm (4.1 cm3 = 4 x 109 cells) tumour but definitely not for 3.9 cm (33 cm3 = 3.3 x 1010 cells) tumour. The TNM stage likely works for surgery (it is not important whether 2 cm or 4 cm tumour is excised) but it is definitively not predictive for individual dose fractionation. There is about 10 fold difference in tumour volume of the smallest and the largest T2 tumour what gives difference of 1 decade (1 log) of tumour cells. Therefore the largest tumours in this category should receive about 7-10 Gy[1]* higher total dose (with no change in the OTT) than the smallest ones. However it did not become a rule in daily practice. Trends of the OTT and initial tumour volume (Tv) are very similar – local tumour control (LTC) dramatically decreases with extension of the OTT (fig. 1) and increase of the Tv.
Altered Radiotherapy – does Holly Grail exist?
During the last 25 years phenomenon of accelerated repopulation (1) of tumour clonogens (significant decrease in the local control (LTC) with extension overall treatment time (fig. 1) has led to more that 40 different clinical trials on altered dose fractionation with more than 20 000 patients involved. However, overall results are rather disappointing. Among 26 well known trials in H&N cancer only 15 were included into meta-analysis (2). Therapeutic gain in 5-year locoregional control (LRC) was 4-6% and 8% in overall survival but mainly in favour of hyperfractionation. It seems very naive to expect any therapeutic benefit using the same dose and fractionation for variety of tumour sites and stages. These studies clearly show that a single altered fractionation regimen can never be a “Holly Grail” for many different tumours, even within the single region, as head and neck, and average results can never be used as optimal predictors to treat individuals.
The CAIR-I and CAIR-II showed about 40% increase in the 5-year LRC for T3-4N0-1M0 oral cavity and oropharyngeal cancer, but in CAIR-II 5-day-concomitant boost has appeared similarly effective as 7-day irradiation. At the first glance one may say that altered radiotherapy generally failed. On the other hand more optimistic conclusion is that there are likely a few “Holly Grails” for more homogenous subset patients when the TNM staging would be supplemented with the volumetric one.
The next practical conclusion is that any extra dose (popularly called “escalation”) together with the respective OTT extension does not produce any gain. Doses of 60 Gy/30 fx in 42 days, 70 Gy/35 fx in 49 days and 80 Gy/40 fx in 56 days are equally effective (fig. 2). Suwiński and Withers (3) have defined this phenomenon as “effect plateau”. Although a universal “Holly Grail” has not be found the OTT should be as short as possible but not less than 4 weeks with no change in total dose, and the treatment should always start on Monday but never completed on Monday.
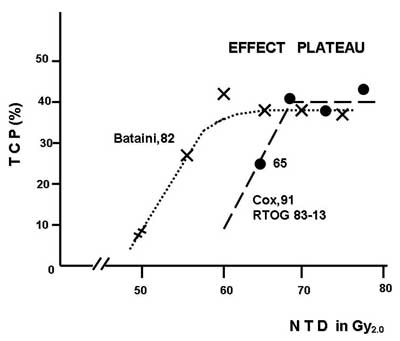
Fig. 2. “Effect plateau” for H & N radiotherapy. Any increase in total dose above 60 Gy with the respective extension of overall treatment time does not produce any therapeutic gain.
HYPO – back to future
At the beginning era of radiotherapy hyperfractionation was the major, and in some countries, the only schedule of radiation dose delivery. It was mainly advocated by Germans and Swedish and was still popular even when such giants as Regaund and Coutard introduced multifractionated treatment. HYPO was abandoned as radical treatment, because of a high risk of serious late complications, and used only as palliation. The comeback of HYPO started in early 50-ties when neuronavigation has developed (stereotaxy). The next step was Gamma Knife and recently Cyber Knife. This technology has led to stereotactic body radiotherapy (SBRT) with large single fractions of 8 Gy to even 22 Gy to relatively small the target volumes. The HYPO offers two major advantages: short time of treatment and short hospitalization and it became the domain of radiobiology (4). According to Fowler, any tumour radioresistance has no chance to be converted into radiosensitivity if a single fraction is given. However, a few fractions can in principle improve radiosensitivity. Moderate HYPO with fraction dose of 4-8 Gy has been widely popularized. In rectal cancer, preoperative 5 x 5 Gy followed by total mesorectal excision resulted in significant therapeutic gain and about 65-75% sphincter preservation. Three fractions of 22 Gy given over a few weeks to T1-2 NSCLC resulted in 95% 2-yr LRC and 56% OS. Partial-breast irradiation with the dose of 34-38.5 Gy in 10 fractions given using 3D conformal radiotherapy after breast-conserving surgery produces none local recurrence and 90-92% excellent cosmetic outcome in the study of Beaumont Centre (4).
Long clinical practice has shown that linear quadratic model and α/β factor likely do not work for doses below 1 Gy and above 8-10 Gy. Ritter and Fowler reported an α/β value lower than 2 Gy for prostate cancer (less than for spinal cord) and it was almost like “October Revolution”. Prostate cancer Immediately became a target for HYPO, and 5 x 5.5-7 Gy have been mandatory employed in the USA and Canada. Martinez introduced 4 x 8.5, 9, 9.5 Gy schedules as a sole treatment and noted acceptable incidence of late effects similar to conventional RT with pronounced benefit in LRC. It was found as a challenge to conventional treatment, and Fuks with Zelefsky from Sloan Kettering Cancer Institute in New York used high-tech 3D-conformal IMRT “dose painting” with 1.8 Gy/fractional given up to 90-92 Gy. Is has been recognized as “High Society” of radiotherapy because treatment planning was based on MRI-PET fusion biological imaging of tumour subvolumes with hypoxia, high proliferation, or cell density, intrinsic resistance and with individual dose painting according to the resistance of subvolumes.
Although the HYPO has made very fast career, the challenge between HYPO, HYPER, Brachytherapy and in the field boost IMRT (SIB-IMRT) continues, and till now there is no clear advantage of one of them.
3D-4D-IMRT, IGRT, SBRT, IART, IORT – series of bank safe codes! which one works?
After disappointment with altered fractionation High-Tech revolution in radiotherapy has appeared as the next promising “Holly Grail”. It offers precise conformal delivery of individually shaped radiation beams focused within the tumour target. Dose can be escalated and normal tissue better protected. Stereotaxy and dose intensity modulation can even be shaped in the tumour in high degree of freedom and with higher doses in some specified subvolumes (IMRT).
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Maciejewski B, Withers HR, Taylor JMG et al.: Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and nasopharynx. Tumour dose-response and repopulation. Int J Radiat Oncol Biol Phys 1989; 16: 831-840.
2. Bourkis J, Overgard J, Andry H et al.: Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006; 368: 843-854.
3. Suwinski R, Withers HR: Time factor and treatment strategies in subclinical disease. Int J Radiat Oncol Biol Phys 2003; 79: 495-502.
4. Thummerman RD. edit. Hypofractionation. Semin Radiat Oncol 2008; 18: 215-265.
5. Wang J, Bai S, Chen N et al.: The clinical feasibility and effect of online cone beam computer tomography – guided intensity – modulated radiotherapy for nasopharyngeal cancer. Radiother Oncol 2009; 90: 221-227.
6. Bonner JA, Harari PM, Giralt J et al.: Radiotherapy plus Cetuximab for squamous cell carcinoma of the head and neck. N Engl J Med 2006; 354: 567-578.
7. Hellman S: Karnofsky Memorial Lecture National History of Small Breast Cancer. J Clin Oncol 1994; 12: 2229-2234.
8. Heiman R, Hellman S: Individual characterization of the metastatic capacity of human breast cancer. Eur J Cancer 2000; 36: 1631-1639.
9. Szala S: Angiogenesis and immune suppression: yin and yang of tumour progression. Post Hig Med Research 2009; 63: 598-612.
10. Van Houten VMM, Leemans CR, Kummer JA et al.: Molecular diagnosis of surgical margins and local recurrence in head and neck cancer patients, a prospective study. Clin Cancer Res 2004; 10: 3614-3620.
11. Braakhuis BJM, Leemans CR, Brakenhoff RH. Expanding fields of genetically altered cells in head and neck squamous carcinogenesis. Semin Cancer Biol 2005; 15: 113-120
12. Kepka L, Maciejewski B, Withers HR: Does incidental irradiation with doses below 50 Gy effectively reduce isolated nodal failure in non-small cell lung cancer: dose-response relationship. Int J Radiat Oncol Biol 2009; 73: 1391-1396.
13. Bao S, Wu Q, Mc London RE et al.: Glioma steam cells presente radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756-760.
14. Suwiński R, Jaworska M, Nikiel B: Predicting effect of accelerated fractionation in postoperative radiotherapy for head and neck cancer based on molecular marker profiles: data from a randomized clinical trial. Int J Radiat Oncol Biol Phys 2010; 77: 438-447.
15. Bentzen SM: From cellular to light-throughput predictive assays in radiation oncology. Challenges and Opportunities. Semin Radiat Oncol 2008; 18: 75-88.
16. Pignon J, Bourhis J, Domenage L et al.: Chemotherapy added to locoregional treatment for head an neck squamous cell carcinoma. Three meta-analyses of updated individual data. Lancet 2000; 335: 940-955.


