Could membrane lipids influence the receptor tyrosine kinase activity? Epidermal growth factor receptor and its interactions with gangliosides**
Czy lipidy błonowe modyfikują aktywność receptorowych kinaz białkowych? Receptor epidermalnego czynnika wzrostu i jego oddziaływania z gangliozydami
Department of Biochemistry and Molecular Biology, Medical Center of Postgraduate Education, Warsaw
Head of Department: prof. dr hab. Barbara Czarnocka
Glycosphingolipids (GSLs) are ubiquitous components of all vertebrate cell membranes. They represent from less than 5% (erythrocytes) to more than 20% (myelin) of membrane lipids (1). Although GSLs have common schematic structure, they are highly heterogeneous group of lipids. With regard to their headgroups, the GSLs were divided into two groups: galactosphingolipids and glucosphingolipids (2). The sialic acid containing glucosphingolipids are the gangliosides (3).
It was observed that GSLs are not uniformly distributed in the membrane. Instead GSLs are clustered in structures called “lipid rafts”, which are defined as “areas in the membrane different in lipid composition from other membrane regions and characterized by a lateral organization dictated by the properties of their lipid composition. This has significant influence on large number of biological events” (4-10).
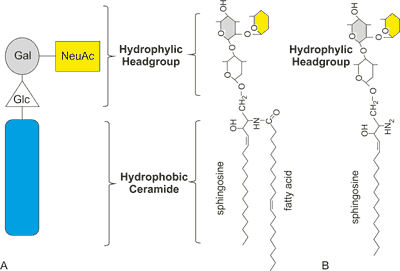
As it was already mentioned GSLs, including gangliosides, are lipid rafts components. Uneven distribution of gangliosides is probably the result of their specific structure. They have a hydrophobic long carbon chain residue (ceramide) and a hydrophilic oligosaccharide moiety (hydrophilic headgroup) (fig. 1A). The long saturated carbon chains of ceramide have unique biophysical properties, which allow self-aggregation and formation of “clusters” (1). The gangliosides hydrophilic oligosaccharides chains provide sites for interaction with extracellular molecules such as toxins, extracellular matrix components, adhesion molecules, receptors and enzymes on the surface of adjacent cells (trans interaction). Moreover gangliosides hydrophilic headgroups take part in lateral interaction (cis interaction) with receptors and enzymes in the same membrane (11).
Fig. 1. Gangliosides and lyso-gangliosides structures: A: GM3 – schematic and structural formula; B: lyso-GM3 structural formula; (Glc – glucose, Gal – galactose, NeuAc – neuraminic acid).
The interaction between gangliosides and proteins in lipid rafts are still not fully understood. It was proven that “cluster” of gangiosides is a less fluid part of the membrane, where membrane associated proteins could be confined. Within the lipid rafts there are lateral interactions between lipids and proteins. But also there are limited interactions between proteins associated with different lipid rafts (12).
It should be mentioned that only some proteins classes are highly concentrated in lipids rafts (13). The most popular motifs present in lipid rafts proteins are a glycophosphatidylinositol anchor or a lipid modifications of protein such as myristoylation or palmitoylation. Also transmembrane proteins are present in lipid rafts despite the modeling of transmembrane peptides that indicated in general that targeting to liquid-ordered phases is disfavored (12). These transmembrane proteins localized in the lipid rafts are growth factor receptors, including receptors for fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), insulin and epidermal growth factor (EGF) (13). It has been assumed that the trapping of certain proteins in lipid rafts might be somehow crucial to their biological role (14).
This hypothesis was confirmed by a wide-range of studies on the role of gangliosides in function of membrane protein receptors. The negative (inhibitory) and positive (activatory) effect of gangliosides on receptor proteins were observed. For example GM1, GM2, GD1a and GT1b cause inhibition of PDGFR phosphorylation (15-16) and GM3 reduces EGFR phosphorylation. In contrast enhancement of growth factor receptor phosphorylation has been observed with GD1a and GM1 for EGFR (17-19) and FGFR (20). The opposite effects were detected as a result of interactions between receptor and gangliosides, thus suggesting that the mechanism of these interactions is complex.
Probably, this situation results from several possibilities of binding between ganglioside molecules and proteins. The first option is the binding between ganglioside and proteins amino acid residues in the extracellular loops. The second is the binding between ganglioside headgroup and sugar residues in glycans of a glycosylated proteins. The last option is the hydrophilic binding with the oligosaccharide part of an anchor of GPI-anchored proteins (12). These three types of binding could induce different conformation changes in membrane protein, which probably would influence their biological functions.
1. Schnaar RL, Suzuki A, Stanley P: Glycosphingolipids. [In:] Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology. 2nd edition. New York: Cold Spring Harbor Laboratory Press 2009.
2. Gault CR, Obeid LM, Hannun YA: An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol 2010; 688: 1-23.
3. Svennerholm L: Composition of gangliosides from human brain. Nature 1956; 177: 524-5.
4. Lajoie P, Partridge EA, Guay G et al.: Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol 2007; 179: 341-56.
5. Guirland C, Zheng JQ: Membrane lipid rafts and their role in axon guidance. Adv Exp Med Biol 2007; 621: 144-55.
6. Benarroch EE: Lipid rafts, protein scaffolds, and neurologic disease. Neurology 2007; 69: 1635-9.
7. Riethmüller J, Riehle A, Grassmé H et al.: Membrane rafts in host-pathogen interactions. Biochim Biophys Acta 2006; 1758: 2139-47.
8. Debruin LS, Harauz G: White matter rafting-membrane microdomains in myelin. Neurochem Res 2007; 32: 213-28.
9. Delacour D, Jacob R: Apical protein transport. Cell Mol Life Sci 2006; 63: 2491-505.
10. Taylor DR, Hooper NM: The prion protein and lipid rafts. Mol Membr Biol 2006; 23: 89-99.
11. Sonnino S, Prinetti A: Gangliosides as regulators of cell membrane organization and functions. Adv Exp Med Biol 2010; 688: 165-84.
12. Prinetti A, Loberto N, Chigorno V et al.: Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta 2009; 1788: 184-93.
13. Kaucic K, Liu Y, Ladisch S: Modulation of growth factor signaling by gangliosides: positive or negative? Methods Enzymol 2006; 417: 168-85.
14. Jacobson K, Mouritsen OG, Anderson RG: Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 2007; 9: 7-14.
15. Farooqui T, Kelley T, Coggeshall KM et al.: GM1 inhibits early signaling events mediated by PDGF receptor in cultured human glioma cells. Anticancer Res 1999; 19: 5007-13.
16. Hynds DL, Summers M, Van Brocklyn J et al.: Gangliosides inhibit platelet-derived growth factor-stimulated growth, receptor phosphorylation, and dimerization in neuroblastoma SHSY5Y cells. J Neurochem 1995; 65: 2251-58.
17. Li R, Liu Y, Ladisch S: Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J Biol Chem 2001; 276: 42782-92.
18. Li R, Manela J, Kong Y et al.: Cellular gangliosides promote growth factor-induced proliferation of fibroblasts. J Biol Chem 2000; 275: 34213-23.
19. Liu Y, Li R, Ladisch S: Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J Biol Chem 2004; 279: 36481-9.
20. Rusnati M, Urbinati C, Tanghetti E et al.: Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc Natl Acad Sci USA 2002; 99: 4367-72.
21. Wang X, Paller AS: Membrane regulation of EGFR signaling by gangliosides. Open Dermatol J 2009; 3: 159-62.
22. Bremer EG, Schlessinger J, Hakomori S: Gangliosidemediated modulation of cell growth: specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem 1986; 261: 2434-40.
23. Hanai N, Nores GA, MacLeod C et al.: Ganglioside-mediated modulation of cell growth. Specific effects of GM3 and lyso-GM3 in tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem 1988; 263: 10915-21.
24. Yednak MA, Bremer EG: Preferential binding of the epidermal growth factor receptor to ganglioside GM3 coated plates. Mol Chem Neuropathol 1994; 21: 369-78.
25. Lambert S, Vind-Kezunovic D, Karvinen S et al.: Ligand-independent activation of the EGFR by lipid raft disruption. J Invest Dermatol 2006; 126: 954-62.
26. Zhou Q, Hakomori S, Kitamura K et al.: GM3 directly inhibits tyrosine phosphorylation and de-N-acetyl-GM3 directly enhances serine phosphorylation of epidermal growth factor receptor, independently of receptor-receptor interaction. J Biol Chem 1994; 269: 1959-65.
27. Carpenter G, King L, Cohen S: Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature 1978; 276: 409-10.
28. Cohen S, Carpenter G, King L: Epidermal growth factor receptor- protein kinase interactions. Prog Clin Biol Res 1981; 66: 557-67.
29. Miljan EA, Meuillet EJ, Mania-Farnell B et al.: Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J Biol Chem 2002; 277: 10108-13.
30. Yoon SJ, Nakayama K, Hikita T et al.: Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci USA 2006; 103: 18987-91.
31. Guan F, Handa K, Hakomori SI: Regulation of epidermal growth factor receptor through interaction of ganglioside GM3 with GlcNAc of N-linked glycan of the receptor: demonstration in ldlD cells. Neurochem Res 2011; DOI 10.1007/s11064-010-0379-9.
32. Kawashima N, Yoon SJ, Itoh K et al.: Tyrosine kinase activity of epidermal growth factor receptor is regulated by GM3 binding through carbohydrate to carbohydrate interactions. J Biol Chem 2009; 284: 6147-55.
33. Kingsley DM, Kozarsky KF, Hobbie L et al.: Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell 1986; 44: 749-59.
34. Weis FMB, Davis RJ: Regulation of epidermal growth factor receptor signal transduction: role of gangliosides. J Biol Chem 1990; 265: 12059-66.
35. Wang XQ, Sun P, Paller AS: Ganglioside induces caveolin-1 redistribution and interaction with the epidermal growth factor receptor. J Biol Chem 2002; 277: 47028-34.
36. Wang X, Rahman Z, Sun P et al.: Ganglioside modulates ligand binding to the epidermal growth factor receptor. J Invest Dermatol 2001; 116: 69-76.
37. Wang XQ, Yan Q, Sun P et al.: Suppression of epidermal growth factor receptor signaling by protein kinase C-alpha activation requires CD82, caveolin-1, and ganglioside. Cancer Res 2007; 67: 9986-95.
38. Li R, Liu Y, Ladisch S: Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J Biol Chem 2001; 276: 42782-92.
39. Liu Y, Li R, Ladisch S: Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J Biol Chem 2004; 279: 36481-9.
40. Miljan EA, Bremer EG: Regulation of growth factor receptors by gangliosides. Sci STKE 2002; 2002: re15.
41. Hofman EG, Ruonala MO, Bader AN et al.: EGF induces coalescence of different lipid rafts. J Cell Sci 2008; 121: 2519-28.
42. Yamabhai M, Anderson RG: Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem 2002; 277: 24843-6.
43. Saffarian S, Li Y, Elson EL et al.: Oligomerization of the EGF receptor investigated by live cell fluorescence intensity distribution analysis. Biophys J 2007; 93: 1021-31.
44. Fujimoto Y, Izumoto S, Suzuki T et al.: Ganglioside GM3 inhibits proliferation and invasion of glioma. J Neurooncol 2005; 71: 99-106.
45. Nishio M, Tajima O, Furukawa K et al.: Over-expression of GM1 enhances cell proliferation with epidermal growth factor without affecting the receptor localization in the microdomain in PC12 cells. Int J Oncol 2005; 26: 191-9.
46. Uemura S, Feng F, Kume M et al.: Cell growth arrest by sialic acid clusters in ganglioside GM3 mimetic polymers. Glycobiology 2007; 17: 568-77.
47. Haga Y, Hakomori SI, Hatanaka K: Quantitative analysis of EGFR affinity to immobilized glycolipids by surface plasmon resonance. Carbohydr Res 2008; 343: 3034-8.
48. Murozuka Y, Watanabe N, Hatanaka K et al.: Lyso-GM3, its dimer, and multimer: their synthesis, and their effect on epidermal growth factor-induced receptor tyrosine kinase. Glycocon J 2007; 24: 551-63.