© Borgis - New Medicine 3/2011, s. 84-89
*Agata Kabała-Dzik1, Anna Rzepecka-Stojko2, Jerzy Stojko3, Ewa Szaflarska-Stojko1, Magdalena Wyszyńska1, Robert Kubina1, Aleksandra Kolarczyk1, Wojciech Marquardt1, Barbara Stawiarska-Pięta1
Evaluation Attempt of Bee Larva DNA Influence on the Course of Rat Pregnancy
1Department and Institute of Pathology, The Faculty of Pharmacy in Sosnowiec, Medical University of Silesia
Head of Department: dr hab. n. med. Barbara Stawiarska-Pięta
2Department of Pharmaceutical Chemistry, The Faculty of Pharmacy in Sosnowiec, Medical University of Silesia
Head of Department: prof. dr hab. n. farm. Ewa Buszman 3Department of Hygiene, Bioanalysis and Environmental Studies, The Faculty of Pharmacy in Sosnowiec, Medical University of Silesia
Head of Department: dr hab. n. med. Jerzy Stojko
Summary
Introduction. Beneficial properties of products collected, processed and secreted by bees, have been known and used for centuries. The latest trend in apitherapy is examination of a possibility to apply bee larva extract in proteinotherapy. The key to success and at the same time the greatest difficulty in experiments regarding a therapy with DNA application, is the use of an appropriate carrier, called vector. The simplest way of genetic material transport is a direct injection into target, uncovered tissues or organs, bare DNA molecules, suspended in a buffer solution.
The aim of the study. The aim of the study was evaluation of bee larva DNA influence upon the course of rat pregnancy, following the administration by intraperitoneal injection. Control group I included 10 rat females, which were administered sodium chloride solution by intraperitoneal injection on day 4, 10 and 14 of pregnancy. Experimental group I included 10 females, which were administered bee larva DNA biopreparation by intraperitoneal route in 50 μg on day 4, 10, 14 of pregnancy.
Material and methods. After the elimination of both animal groups on 21st day of pregnancy, zootomic examinations of mothers and fetuses were performed, including: macroscopic inspection of females′ internal organs, evaluation of culture parameters of females, external inspection of rat fetuses and morphometric measurements of their bodies and evaluation of the number of ossification points in fetuses.
Results and conclusions. The analysis of the results obtained in both examined groups indicates, that bee larva DNA does not cause pathologic changes within internal organs of pregnant female rats, does not cause defects in fetuses′ development and also does not contribute to the occurrence of congenital defects in fetuses.
Introduction
In recent years there has been a renaissance of natural medicine, a large interest in pharmacopeial items, hence, apitherapy appears as a major alternative for a variety of synthetic therapeutics.
The latest studies confirm cytoprotective action of apipharmacotherapeutics in the course of anticancer treatment. In the studies concerning hepatic tissue and crosswise-striped cardiac tissue in rats, a protective action of propolis extracts was shown in relation to these tissues, manifested in the reduction of defects resulting from oxidative stress (1-8).
In recent time, scientists have been interested in the possibility of introducing a new raw material into apitherapy, which is bee larva. Although gene therapy appears to be a promising perspective in the treatment of various ailments, some limitations impede its wide application. These matters are mainly connected with a lack of a universal carrier, with a possibility of a strong immunologic response induction by means of nucleic acids administered into the body, or achieving an appropriately long-term therapeutic action. The key to success, and at the same time the greatest difficulty in experiments concerned with gene therapy is the use of an appropriate carrier i.e., vector. One of the methods is injecting bare, uncovered DNA molecules, suspended in a buffer solution into pathologically altered tissues or organs (9-15). Developing fetus is extremely sensitive to destructive action of toxic substances, due to the lack of its own biotransformation systems, and therefore, totally dependent on the mother and exposed to all substances in her body, capable of crossing placental barrier. Due to this fact, intraperitoneal administration of bee larva DNA extract to protect the fetus against environmental toxins must be preceded by an accurate analysis of the effect of this substance upon mother′s body and developing fetus, along with confirmation and documentation of its efficacy and safety in use.
Aim OF THE STUDY
The aim of the study was evaluation of bee larva DNA influence on the course of pregnancy in female rat after administration of this substance by intraperitoneal route. It was achieved by answering the following questions:
– What are the effects of bee larva DNA upon pregnant female rats?
– Has bee larva DNA any influence on morphometric parameters of rat fetuses?
– How does bee larva DNA affect osteoarticular system of a fetus?
MATERIAL AND METHODS
In the experiment were used 20 pregnant female Wistar rats, approximately 3 months old, with body mass 170 ± 20 g, from the Experimental Medicine Culture Centre of the Medical University of Silesia. The experiment was approved by Ethics Commission (agreement no.: 34/4, 9th of November 2004). The research was financed by Medical University of Silesia (no, KNW-2-015/09).
Rats were divided into 2 groups of 10 animals: control group C – the female rats which were administered physiological saline in a form of intraperitoneal injection, on day 4, 10 and 14 of pregnancy.
Experimental group I – the female rats, which were administered bee larva DNA extract of 50 μg as a suspension in physiological saline (1 ml) in a form of intraperitoneal injection on day 4, 10 and 14 of pregnancy.
Bee larva DNA biopreparation was made for experimental purposes at the department and Faculty of Molecular Biology of Medical University of Silesia in Katowice, in accordance with the standard DNA isolation procedure (15).
On 21st day of pregnancy the rats were anesthetized by intraperitoned administration of morbital at a dose of 200 mg/kg body weight and the following examinations were performed, including:
– Zootomic examination of rat mother, upon which macroscopic inspection of particular organs was made and the following were determined:
• number of fetuses in each of uterus horns;
• number of visible focci of fetuses resorption;
• number of corpuses luteum (yellow bodies) in each ovary;
– morphometric measurements of fetus body:
• weighing a fetus along with placenta;
• weighing a fetus without placenta;
• measurement of a fetus length from mouth to tail base;
• measurement of a fetus length from mouth to tail′s end;
• inspection of all fetuses in order to identify possible congenital defects;
• exposing all ossification points of fetuses by means of Mocca-Dowson′s method;
• counting ossification points in metacarpus, metatarsus and sternum of fetuses.
Statistical analysis
Statistical analysis was performed with SPSS Statistics 17.0. Mean, and SD values were used as the indicators of descriptive statistics. The comparisons between groups were made with Student′s t- test. To check normality of data obtained in the research Shapiro-Wilk test had been used for each distribution of results.
The second point was to use standard Student′s t-test to compare two sets of quantitative data (corresponding distributions). All assumptions were met: samples had been collected independently of one another and all sets have had normal distribution.
Results and discussion
Zootomic examinations of rat mothers
Upon examination, both in a control group, receiving physiological saline (group C) by intraperitoneal route, and in experimental group receiving bee larva DNA (group I) by the same route, no pathological changes in organs of pregnant female rats were found, whereas in all cases normal, developing and advanced pregnancy was confirmed.
Evaluation of culture parameters
Analysis of culture parameters of examined animals is aimed at verification of the influence of exogenous substance which is bee larva DNA extract, administered to female rats, on their fertility.
The number of fetuses in the uterus of individual females ranges 8 – 13. Mean fertility in this animal group amounts to 10.6±1.90. In 2 females 1 visible focus of fetus resorption in the uterus was found, which gives a mean of 0.2±0.42 for this group. The number of corpuses luteum (yellow bodies) in both ovaries in individual females ranged 8-14. Mean theoretical fertility amounts to 10.7±2.06
The results obtained in this part of the experiment made it possible to determine a relation between the substance given to animals and their fertility. The animals used in the experiment are subpopulation of Wistar SM rat, in which a mean fertility is 9. Thus, the obtained results were referred to the standard set for Wistar SM rats. In the examined group I, mean fertility of females increased into 11.1±3.35. This result suggests that bee larva DNA administered to animals does not affect a decrease of their fertility. Focci of fetus resorption reflect the number of necrotic embryos in time of pregnancy. Counting them in the course of the experiment allows ruling out or confirming possible fetotoxic effects of the substance administered to animals. The standard for Wistar SM rats has a value of 1.0. In the experiment, for females receiving bee larva DNA extract, this value is 0.1±0.32 – it is a few times lower than the value in the control group, which allows to rule out toxic effects of administered substance on rat fetuses.
All obtained data show that with confidence level 0.05 the null hypothesis cannot be rejected, therefore it means, that there is no statistical difference between corresponding sets of samples. In conclusion we can say that applying of bee larva DNA hasn′t got toxic properties (any different effects of using bee larva DNA and sodium chloride upon pregnant female rats) (fig. 1-3).
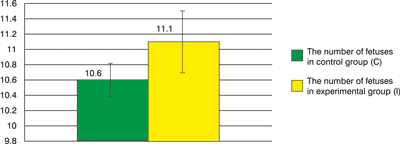
Fig. 1. The number of fetuses in the uterus in both groups.
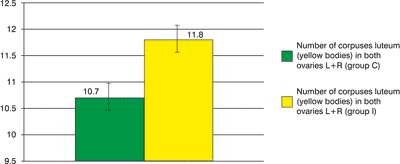
Fig. 2. The number of corpuses luteum in both groups.

Fig. 3. The number of visible focus of fetus resorption in the uterus.
Morphometric measurements of fetuses′ bodies
Results of measurements of fetuses body mass with and without placenta, and also measurements of body length from mouth to tail base and from mouth to tail′s end were given fugures.
In the control group C mean value of fetuses mass with placenta was a bit greater than in the experimental group I, applied by DNA. There wasn′t observed any significant change in the mass of fetuses (without placenta) in the control group I where the bee larva DNA has been applied (fig. 4).
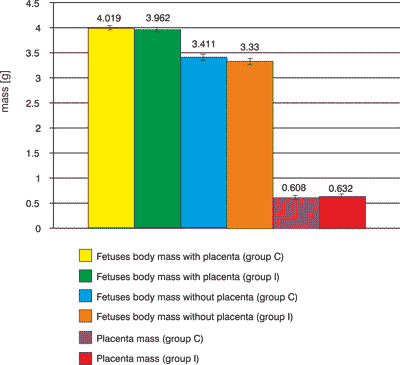
Fig. 4. Fetuses body mass with and without placenta, placenta mass.
There were any significant statistically changes between length of fetuses of females where the bee larva DNA was applied and length of fetuses of females of control group as well. The slightly longer (by 2.73 mm) fetuses (included end of tail) in the group I than in control group C was observed. The length of fetuses from mouth to tail′s base had a mean value 38.10±1.66 mm. The length of fetuses from mouth to tail′s end, the mean value, was 48.52±1.57 mm. Body length of fetuses in this group, from mouth to tail′s base had a mean value 39.45±2.63 mm. however, the body length of fetuses from mouth to tail′s end had a mean value 51.25±2.39 mm (fig. 5).
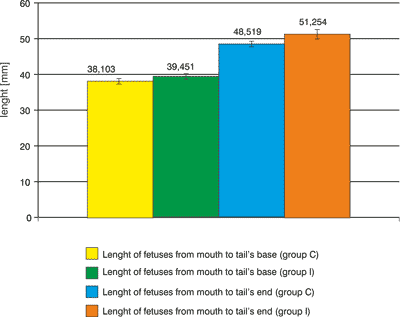
Fig. 5. Lenght of fetuses.
In experimental group, mean fetus length, measured from mouth to tail′s base is 1.35 mm longer than analogous fetus length in a control group, whereas the measured length from mouth to tail′s end is 2.73 mm longer.
The above observations indicate, that bee larva DNA preparation, administered to pregnant females by intraperitoneal route, does not cause disturbances in fetus development.
External inspection of rat fetuses
Upon inspection of rat fetuses, the focus was on body shape, colour of skin coverings, their continuity and possible macroscopic changes or fetus congenital defects. In fetuses from a control group, there were no abnormalities in body structure. In this group, the inspection was performed on 111 fetuses from 10 females, which were administered bee larva DNA biopreparation during pregnancy. None of fetuses in the examined group (I) had deviation from normal condition of body structure. Inspection results indicate, that bee larva DNA extract, administered to pregnant females, does not cause developmental anomalies in fetuses.
Evaluation of ossification points number in fetuses
In control group ossification points were counted in 106 fetuses from females which received physiological saline during pregnancy. The sum of ossification points in the area of metacarpus, metatarsus and sternum of fetuses, amounts to 11.7. In experimental group I, ossification points in 111 fetuses were analysed, from females which received bee larva DNA biopreparation during pregnancy. In fetuses of this group, a mean number of ossification points in metacarpus is 319. A mean number of ossification points in metatarsus of examined fetuses is 4.08. In the area of sternum, a mean number of ossification points is 4.47. The sum of mean numbers of ossification points in metacarpus, metatarsus and sternum in fetuses in experimental group I is 0.01 lower than analogous value for fetuses in a control group (fig. 6).
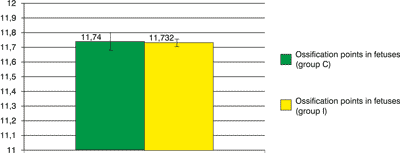
Fig. 6. Ossification points in fetuses.
All statistical methods show the ossification points number in each series (groups C and I) are belonging to the same distribution, so we can statistically say that DNA of bee larva hasn′t got worse influence than natrium chloride on the ossification points in fetuses.
Conclusions
The results obtained in this experiment prove, that administration of bee larva DNA extract to pregnant female rats, does not disturb the course of pregnancy, does not cause congenital defects in fetuses, its action does not damage a skeleton and does not contribute to disturbances in fetus development.
Piśmiennictwo
1. Stojko A: Czerw pszczeli nowym surowcem farmakopealnym. Materiały z Naukowej Konferencji Pszczelarskiej, Puławy 2005, http://www.opisik.pulawy.pl/pdf/matXLII.pdf, www.opisik.pulawy.pl. 2. Benguedouar L, Boussenane HN, Wided K et al.: Efficiency of propolis extract against mitochondrial stress induced by antineoplasic agents (doxorubicin and vinblastin) in rats. Indian J Exp Biol 2008; 2: 112-9. 3. Temiz M, Aslan A, Canbolant E et al.: Effect of propolis on healing in experimental colon anastomosis in rats. Adv Ther 2008; 28: 412-21. 4. Couteau C, Pommier M, Paparis E et al.: Photoprotective activity of propolis. Nat Prod Res 2008; 22: 264-8. 5. Inokuchi I, Shimazawa M, Nakajima Y et al.: Brazilian Green Propolis protects against Retinal Damage in vitro and in vivo. Evid-Based Compl Alt Med 2006; 3: 71-7. 6. Weinstock GM, Robinson GE, Gibbs RA et al.: Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006; 443(7114): 931-49. 7. Kabała-Dzik A, Szaflarska-Stojko E, Wojtyczka R et al.: Efficiency assessment of antimicrobial activity of honey-balm on experimental burn wounds. Bul Vet Inst Pulawy 2004; 48: 109-12. 8. Evans JD, Aronstein K, Chen YP et al.: Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 2006; 15: 645-56. 9. Anson DS: The use of retroviral vectors for gene therapy – what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Gene Vac Ther 2004; 2: 9-15. 10. Relph K, Harrington K, Pandha H: Recent developments and current status of gene therapy using viral vectors in the United Kingdom. Br Med J 2004; 329: 839-42. 11. Glasgow JN, Everts M, Curiel DT: Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther 2006; 13: 830-844. 12. Coura dos Santos R, Beyer Nardi N: The state of the art of adeno-associated virus-based vectors in gene therapy. Vir J 2007; 4: 99. 13. Goverdhana S, Puntel M, Xiong W et al.: Regulatable Gene Expression Systems for Gene Therapy Applications: Progress and Future Challenges. Curr Gene Ther 2006; 6: 421-38. 14. Helenius E, Boije M, Niklander-Teeri V et al.: Gene delivery into intact plants using the Helios Gene Gun. Plant Mol Biol Reptr 2000; 18: 287a-287l. 15. Barki Y, Douek J, Graur D et al.: Polymorphism in soft coral larvae revealed by amplified fragment-lenght polymorphism (AFLP) markers. Mar Biol 2000; 136: 37-41.





