© Borgis - Postępy Nauk Medycznych 12/2011, s. 1054-1060
*Joanna Wójtowicz1,2, Joanna Leszczyńska2, Katarzyna Walenko2, Małgorzata Lewandowska-Szumieł2
Produkty inżynierii tkankowej dla pacjentów pediatrycznych
Tissue engineered products for pediatric patients
1Clinical Department of Pediatrics, Bielanski Hospital, Warsaw, Poland
Head of the Pediatric Department: prof. Teresa Jackowska, MD, PhD
2Department of Biophysics and Human Physiology, Medical University of Warsaw, Poland
Head of Department: prof. Jacek Przybylski, MD, PhD
Streszczenie
Inżynieria tkankowa to nowa dziedzina medycyny regeneracyjnej, zajmująca się możliwościami regeneracji tkanek przy pomocy produktów leczniczych zawierających komórki z hodowli in vitro. Zwykle komórki przeznaczone do transplantacji są osadzone na podłożu biomateriałów, często z dodatkiem czynników stymulujących wzrost tkanki. Produkty inżynierii tkankowej (PIT) są alternatywą dla obecnie stosowanych metod rekonstrukcji tkanek – tj. przeszczepów autologicznych, allogenicznych lub implantów z materiałów syntetycznych.
Potrzeba regeneracji tkanek u dzieci pojawia się w przypadku niektórych wad rozwojowych. Występuje także wówczas, gdy potrzebne jest zaopatrzenie ubytków powstałych w wyniku urazu lub interwencji chirurgicznej, np. u pacjentów onkologicznych. Potencjalna przewaga produktów inżynierii tkankowej nad rozwiązaniami dostępnymi obecnie u pacjentów pediatrycznych polega między innymi na tym, że procesowi wzrastania i rozwoju młodego organizmu powinna towarzyszyć przebudowa przeszczepu. Pozwala to uniknąć wymiany przeszczepu w trakcie rozwoju pacjenta.
Dostępne są już komercyjnie produkty inżynierii tkankowej do regeneracji skóry, tkanki kostnej i chrzęstnej. Trwają prace nad wprowadzeniem na rynek kolejnych substytutów tkanek, jak np. naczyń krwionośnych, zastawek serca, wysepek trzustkowych. Wiele doniesień naukowych zawiera opis eksperymentalnego wykorzystania produktów inżynierii tkankowej u pacjentów pediatrycznych. Są one przygotowywane „na miarę” z komórek własnych pacjenta, biomateriałów i czynników stymulujących regenerację i wzrost odtwarzanej tkanki.
Artykuł zawiera podstawowe informacje o założeniach inżynierii tkankowej, wykorzystywanych metodach oraz dostępnych produktach komercyjnych. Przedstawiono też opis klinicznego zastosowania produktów u pacjentów pediatrycznych w celu regeneracji skóry i naskórka, tętnicy płucnej, plastyki pęcherza moczowego i cewki moczowej oraz rekonstrukcji kości.
Summary
Tissue Engineering, a new field of regenerative medicine, offers products for tissue regeneration obtained by means of cell culture, biomaterials and factors which stimulate tissue growth. Tissue engineered products (TEPs) are an alternative to the methods currently used for tissue regeneration, e.g. transplantation of autogenic or allogenic tissues, or implantation of synthetic materials.
The need for tissue regeneration in children results from congenital malformations, traumas or defects caused by surgical intervention, e.g. tumor resections. One of the potential advantages of tissue engineered products over classical methods for tissue regeneration in children is related to the feature that implants obtained in vitro, after their implantation, should participate in the process of growth of the young organism (and a rebuilding of the graft is expected to occur). Therefore, no morbidity occurs, as opposed to the implant exchange otherwise conventionally performed due to the patient’s development.
There are several tissue engineered products of bone, cartilage and skin on the market already. Research is carried out on launching other tissue substitutes on the market, such as vessels, valves or pancreatic islets. Many scientific reports are published about the experimental use of tissue engineered products in pediatric patients with TEPs made from autologous cells, biomaterials and factors stimulating regeneration and growth of the reconstructed tissue.
The article presents basic information about tissue engineering, its methods and products already present on the market, followed by a description of the regeneration of skin and epidermis, pulmonary artery, bladder, urethra and bone reconstruction in children.

The regeneration and reconstruction of tissues and organs is a current clinical issue in pediatrics. The need to restore or augment the function of tissue applies to all organs; in children it may be caused by congenital malformations, traumas or may result from a treatment e.g. after a tumor resection (1). A variety of sizes and shapes of reconstructed tissues should be taken into account. Moreover, one should aim to achieve the ability of the graft to adjust to the child’s constant growth and development in terms of the graft’s structure and function.
In this context, the clinical methods of tissue reconstruction in children used currently are not fully satisfying. Three methods are applied to regenerate tissue defects: autologous tissue transplantation (e.g. parts of bone or vessels), allogenic transplantation (e.g. biostatic grafts from cadavers) or implanting synthetic materials.
An autologous transplantation is the “golden standard”, as the patient’s own biological materials is the best for tissue regeneration. Autologous grafts induce tissue formation of the recipient. However, using this method is restricted by the limited resources of healthy tissue in the patient, and it is not recommended in the case of young patients in the period of their growth and development. The next method is an allogenic tissue transplantation, mainly in the form of biostatic implants prepared at tissue banks. The preparation of such implants requires the usage of highly-specialized procedures, such as implant fixing and sterilization. A serological examination of the donor tissue should also be provided in order to eliminate the risk of disease transmission. Moreover, the progress of this method is limited by the too low number of tissue donors. The alternative to the autologous or allogenic transplantation is the use of synthetic biomaterials. There is a wide variety of biomaterials prepared from metals, polymers and ceramics, as well as their composites. Unfortunately, the materials do not guarantee a regeneration of the tissue, and are often not able to integrate with recipient’s tissues.
There is an intense need to find alternative, innovative methods to cure tissue defects. Due to this fact, we observe fast progress of a new field of regenerative medicine, i.e. tissue engineering.
Tissue engineering
Tissue engineering is an interdisciplinary field which links engineering, natural and medical sciences in order to obtain biological substitutes to regenerate, maintain or augment the functions of tissue (2). The developments of tissue engineering are already applied in therapy and diagnostics. The field is based on the assumption that three components are needed for tissue regeneration: scaffolds, stimulating factors, and cells (fig. 1).
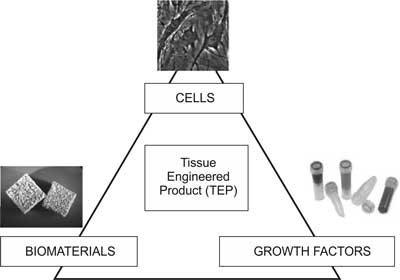
Fig. 1. Components of tissue engineered products.
The first component of a tissue engineered product is a scaffold, which may be made of a natural or synthetic biomaterial. The role of the scaffold is to fill up the tissue defect, offer mechanical support for cells, and provide a proper environment of cell proliferation and differentiation. The material of the scaffold should therefore be biocompatible, bioresorbable, and of the required mechanical properties (3). The idea is that the scaffold implanted into the defect, after enabling the cells to proliferate, should gradually degrade, and be replaced by living tissue. The scaffold may be produced from synthetic polymers, such as poly-L-lactate (PLLA), polyglicolide (PLGA), poly(?-caprolactone) or of polymers of a natural origin, such as collagen, alginate or chitosan (4). Ceramic materials, mainly calcium phosphates, and natural bone matrix in the form of fresh autologous bone or a demineralised matrix, are also taken into account.
The second component of the triad are stimulating factors. The formation of each tissue demands a microenvironment which would provide proper factors to stimulate cell proliferation, morphogenesis and differentiation. It is proven that growth factors play a crucial role in tissue regeneration. Thus, biomaterials which contain proper growth factors have recently been produced (5-7). Besides, the scaffold may also be enriched with proteins (e.g. fibronectin, integrins), hormones or vitamins.
The last component of the product are living cells. Three main types of cells are used in tissue engineering: autologous cells (which offer immunological compatibility), allogenic cells (which may be rejected by the recipient’s body) and xenogenic cells (which may be rejected, and additionally carry the risk of an animal disease transmission). Nowadays, somatic (8, 9) and embryonic stem cells (10, 11), as well as induced pluripotent cells (12, 13) are of interest here.
The preparation of cells for transplantation in the form of a tissue engineered product requires proper cell isolation from tissues obtained from the donor, which is followed by cell proliferation in vitro in order to multiply the cell number. The next step is to place the cells on a three-dimensional scaffold, followed by a cell culture in bioreactors, which are specialized devices for cell culture, providing a proper microenvironment and distribution of the growth factors. The construct, which is obtained in the above manner, is then transplanted into the tissue defect, where the cells build new tissue, and the biomaterial gradually degrades (fig. 2).
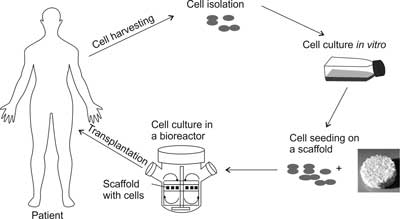
Fig. 2. The stages of preparing of a tissue engineering product (TEP).
The advantage of using tissue engineering products results from combining the knowledge and practice of autologous and allogenic transplantation with the possibilities made available by using artificial material implants. In the case of using patient’s own cells, there is no risk of immunological incompatibility, infection or disease transmission from the donor to the recipient. The tissue engineered product is autologous, but – contrary to the common practice in autologous transplantation – the amount of the patient’s tissue necessary to produce the graft is very little. The amount of the biological material may be increased in vitro, and the product may therefore be tailored for the particular patient’s needs.
Tissue engineered products, however, are not free of disadvantages. The main limitation is that, due to the fact that the product is tailor-made, it is sometimes impossible to increase the production, as the majority of the laboratory-invented products will not reach serial production. Additionally, the fact that the composition includes viable cells complicates the way the products are prepared, stored and transported. Thus, they are not “off-the-shelf” products, which means that there is a time gap between the time when the tissue is collected from the donor and the time when the product is ready to be implanted into the recipient’s body. This time gap may last even several weeks, and it is impossible to provide the treatment at once.
The needs for tissue regeneration in children
There are many children’s tissues whose regeneration by the means of tissue engineering would be an advantageous clinical alternative to the current methods. Not only defect regeneration, but also restoring the given organ’s physiological function is possible to be provided (e.g. implanting active pancreatic islets releasing insulin).
There are several tissue substitutes for children which are of interest at the laboratories of universities or companies. Among them there are substitutes of:
– skin (necessary after burns or to reconstruct defects e.g. in the vicinity of spina bifida defects),
– bone and teeth (reconstructions of auditory ossicles, bone loss after tumor resections or after trauma),
– cartilage (e.g. in the case of chondromalacia, aseptic necrosis, to reconstruct nose cartilage or ear conch),
– tendons and ligaments (after traumas or to reconstruct tissue during orthopedic surgeries of limb malformations),
– vessels (to reconstruct congenital vessel malformations or after a trauma),
– elements of the respiratory system (to reconstruct congenital malformations of e.g. trachea or bronchia),
– liver (to reconstruct e.g. bile ducts)
– pancreas (in e.g. in diabetes – pancreatic islets releasing insulin),
– bladder, ureters and urethra (to reconstruct the organs with congenital or developmental malformations).
Commercially available tissue engineered products
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Saxena, AK: Tissue engineering and regenerative medicine research perspectives for pediatric surgery. Pediatr Surg Int 2010; 26: 557-573.
2. Langer R, Vacanti JP: Tissue engineering. Science 1993; 260: 920-926.
3. Grolik M, Fiejdasz S: Materiały dla inżynierii tkankowej. 2008; 59-64.
4. Langer R, Tirrell DA: Designing materials for biology and medicine. Nature 2004; 428: 487-492.
5. Dodla MC, Bellamkonda RV: Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 2008; 29: 33-46.
6. Levenberg S, Burdick JA, Kraehenbuehl T, Langer R: Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng 2005; 11: 506-512.
7. Midha R, Munro CA, Dalton PD et al.: Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg 2003; 99: 555-565.
8. Cowan CM, Shi YY, Aalami OO et al.: Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 2004; 22: 560-567.
9. Wu Y, Chen L, Scott PG, Tredget EE: Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007; 25: 2648-2659.
10. Levenberg S, Huang NF, Lavik E et al.: Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA 2003; 100: 12741-12746.
11. Guenou H, Nissan X, Larcher F et al.: Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009; 374: 1745-1753.
12. Martinez-Fernandez A, Nelson TJ, Yamada S et al.: Ips programmed without c-myc yield proficient cardiogenesis for functional heart chimerism. Circ Res 2009; 105: 648-656.
13. Li W, Wang, D, Qin J et al.: Generation of functional hepatocytes from mouse induced pluripotent stem cells. J Cell Physiol 2010; 222: 492-501.
14. Regulation (ec) no 1394/2007 of the european parliament and the council on advanced therapy medicinal products and amending directive 2001/83/ec and regulation (ec) no 726/2004. Official Journal of the European Union (2007).
15. Meyer U, Meyer T, Handschel J, Wiesmann HP: Fundamentals of tissue engineering and regenerative medicine. Springer 2009; 331, 603-607, 686-690, 720.
16. Lanza R, Langer R, Vacanti J: Principles of tissue engineering, 3rd edition. 2007: Elsevier.
17. Husing B, Buhrlen B, Gaisser S: Human tissue engineered products – today’s markets and future prospects, final report for work package 1: Analysis of the actual market situation – mapping of industry and products. 2003, Fraunhofer Institute for Systems and Innovation Research.
18. Http://www.Impomed.Com.Pl/. (cited 19-05-2011).
19. Hohlfeld J, de Buys Roessingh A, Hirt-Burri N et al.: Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005; 366: 840-842.
20. Llames S, Garcia E, Garcia V et al.: Clinical results of an autologous engineered skin. Cell Tissue Bank 2006; 7: 47-53.
21. Atala A, Bauer SB, Soker S et al.: Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006; 367: 1241-1246.
22. Matsumura G, Hibino N, Ikada Y et al.: Successful application of tissue engineered vascular autografts: Clinical experience. Biomaterials 2003; 24: 2303-2308.
23. Shinoka T, Ikada Y: Transplantation of a tissue-engineered pulmonary artery. N Eng J Med 2001; 344: 532-533.
24. Naito Y, Imai Y, Shin’oka T et al.: Successful clinical application of tissue-engineered graft for extracardiac fontan operation. J Thorac Cardiovasc Surg 2003; 125: 419-420.
25. Quarto R, Mastrogiacomo M, Cancedda R et al.: Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001; 344: 385-386.
26. Hibi H, Yamada Y, Ueda M, Endo Y: Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg 2006; 35: 551-555.
27. Lee J, Sung HM, Jang JD et al.: Successful reconstruction of 15-cm segmental defects by bone marrow stem cells and resected autogenous bone graft in central hemangioma. J Oral Maxillofac Surg 2010; 68: 188-194.


