© Borgis - Postępy Nauk Medycznych 10/2016, s. 787-794
*Dariusz Boroń1, Małgorzata Kasprzyk-Żyszczyńska2, Krzysztof Januszyk1, Bogusław Czerny3, Adam Kamiński4, Agnieszka Seremak-Mrozikiewicz5, 6, Andrzej Klejewski7, 8
The role of inflammatory factors in pathogenesis of postmenopausal osteoporosis
Rola czynników zapalnych w patogenezie postmenopauzalnej osteoporozy
1High School of Strategic Planning in Dąbrowa Górnicza
Head of High School: Professor Janusz Bohosiewicz, MD, PhD
2Department Lung Diseases and Rehabilitation, Regional Hospital of Lung Diseases and Rehabilitation in Jaroszowiec
Head of Department: Krzysztof Barczyk, MD, PhD
3Department of General Pharmacology and Pharmacoeconomics, Pomeranian Medical University in Szczecin
Head of High School: Professor Bogusław Czerny, MD, PhD
4Department of Pediatric Orthopaedics and Traumatology, Independent Public Teaching Hospital No 1. Pomeranian Medical University in Szczecin
Head of Department: Professor Maciej Kołban, MD, PhD
5Division of Perinatology and Women’s Diseases, Poznań University of Medical Sciences
Head of Division: Professor Krzysztof Drews, MD, PhD
6Department of Pharmacology and Phytochemistry, Institute of Natural Fibers and Medicinal Plants, Poznań
Head of Department: Professor Bogusław Czerny, MD, PhD
7Department of Nursing, Poznań University of Medical Sciences
Head of Department: Professor Jacek Brązert, MD, PhD
8Department of Obstetrics and Women’s Diseases, Poznań University of Medical Sciences
Head of Department: Professor Jacek Brązert, MD, PhD
Streszczenie
Osteoporoza jest chorobą metaboliczną polegającą na postępującym ubytku masy kostnej, spowodowanym utratą związków wapnia, powiązanym ze zmianami hormonalnymi oraz czynnikami genetycznymi. Osteoporoza trzykrotnie częściej występuje u kobiet, co jest związane ze zmianami hormonalnymi towarzyszącymi okresowi menopauzy. Obniżanie się masy szkieletu kostnego podczas starzenia się organizmu spowodowane jest między innymi: zaburzeniem jelitowej absorpcji wapnia, spadkiem poziomu aktywnej witaminy D, wzrostem poziomu parathormonu, wzrostem aktywności osteoklastów oraz obniżeniem poziomu estrogenów, co aktywuje proces resorpcji kości. Osteoporoza rozwija się pod wpływem interakcji pomiędzy czynnikami środowiskowymi, hormonalnymi i genetycznymi, które oddziałują na wartość BMD (gęstość mineralna kości) oraz wpływają na ryzyko złamań. Środowiskowymi czynnikami ryzyka rozwoju tej choroby są czynniki żywieniowe: niedożywienie, niedostateczna podaż wapnia czy witaminy D, styl życia, w tym brak aktywności fizycznej, stosowanie używek, liczba przebytych ciąż, przebyte choroby, zaburzenia dotyczące szpiku kostnego, przebyte stany zapalne czy zażywane leki. Odkrycie trzech podstawowych cząsteczek, które sterują pracą osteoklastów, a tym samym wpływają na osteoporozę i inne choroby kości, było punktem zwrotnym, który rozpoczął nową erę w medycynie. Te cząsteczki to aktywator receptora jądrowego czynnika κB (RANK), jego ligand RANKL oraz naturalny receptor pułapka dla RANKL, czyli osteoprotegeryna (OPG).
Wiadomo, że osteoporoza jest chorobą uwarunkowaną występowaniem licznych polimorfizmów tzw. „genów kandydujących” do jej rozwoju. W badaniach na ten temat ocenia się zmienność tych genów w populacjach ludzkich, opierając się przy tym na identyfikacji polimorfizmów pojedynczego nukleotydu (SNP). Polimorfizmy te mogą wpływać na zmienność takich cech, jak gęstość mineralna kości.
Wśród regulatorów szlaku sygnalizacyjnego przemiany kostnej podkreśla się rolę cytokin, które mogą wspierać lub hamować resorpcję kości. Interleukina IL-17 czy TNF-α zwiększają ekspresję RANKL i dlatego uznaje się je za czynniki proresorpcyjne, stymulujący osteoklastogenezę.
Summary
Osteoporosis is a metabolic disease where progressing loss of the bone mass effects from the loss of calcium compounds, associated with hormonal changes and genetic factors. Osteoporosis is three times more frequent in women which is associated with hormonal changes throughout the menopausal period. Reduced mass of the bone skeleton during ageing is caused, among other, by impaired intestinal absorption of calcium, decreased level of active vitamin D, elevated parathormone, increased activity of osteoclasts and reduced level of estrogens which activates bone resorption. Osteoporosis develops as the result of interaction between the environmental, hormonal and genetic factors which affect the bone mineral density and has a role in the risk of fracture. The environmental risk factors include the nutritional patterns: undernutrition, insufficient supply of calcium or vitamin D, lifestyle, including lack of physical activity, the use of substances, the number of pregnancies, the diseases suffered from, disorders of the bone marrow, any past inflammatory conditions or the drugs taken. Discovery of three major particles controlling osteoblasts, therefore influencing osteoporosis and other bone conditions was a turning point to start a new era of medicine. The particles are activators of the receptor of nuclear factor κB (RANK), its ligand RANKL and the natural receptor trap for RANKL, i.e. osteoprotegerin (OPG). Osteoporosis is known to be conditioned by a number of polymorphisms, the so-called “candidate genes” for its development. The studies of the condition evaluate variability of such genes across populations, based on identification of polymorphisms of a single nucleotide (SNP). The polymorphisms may influence variability of such traits, e.g. bone mineral density. Among regulators of the signaling pathways of bone remodeling, the role of cytokines has been emphasized for their capacity to enhance or to inhibit bone resorption. Interleukin IL-17 or TNF-α increase RANKL expression, therefore they are regarded as proresorptive factors, stimulating osteoclastogenesis.

Osteoporosis is a metabolic disease where progressing loss of the bone mass effects from the loss of calcium compounds, associated with hormonal changes and genetic factors. It is characterized by impairments of bone microarchitecture and the osseous tissue metabolism. Misdiagnosing and delayed treatment, both contribute to frequent fractures bringing dangerous consequences. Osteoporosis appears as a growing social issue, therefore it has been classified by the World Health Organisation as a civilization disease.
Today WHO defines the condition as a generalized metabolic disease of bones, characterized by low bone mass, impaired microarchitecture of bones, and in consequence, increased fragility and susceptibility to fractures. The definition of osteoporosis has been complemented by the value of T-score (SD – standard deviation from the average scores for the peak bone mass at the age 20-29 years). It has been accepted that the T-score value indicative of osteoporosis is below 2.5 SD from the average scores for the peak bone mass at the age 20-29 years (1).
The present number of inhabitants of Europe and the United States affected by osteoporosis exceeds 75 million, contributing to more than 2.5 million bone fractures each year. Contrary to Caucasian population, the prevalence of the condition in Afro-Americans is much lower. Osteoporosis is three times more frequent in women which is associated with hormonal changes throughout the menopausal period. Men show the bone mass higher than women, therefore significant loss of the bone mass in them is less frequent. Statistical data on osteoporotic fractures are alarming. Fractures of the hip bone in white women at advanced age have been estimated at least at 3% in year (2).
Defining the ageing process one should observe that it is characterized by reduced general physiological efficiency of the body, where katabolic changes predominate the anabolic ones and susceptibility to illness grows for both, acute and chronic conditions (3).
Ageing is also associated with structural changes within a bone, both a dense and a spongy one. Reduced mass of the bone skeleton during ageing is caused, among other, by impaired intestinal absorption of calcium, decreased level of active vitamin D, elevated parathormone, increased activity of osteoclasts and reduced level of estrogens which activates bone resorption (4).
A breakthrough in woman’s life is the perimenopausal period and the menopause itself. This time is defined as a stage characterized by gradual extinction and eventual exclusion of the ovarian function and regression of menstruation. Throughout that time the female body adapts to altered conditions associated with the lack of hormonal activity of the ovaries, the deficiency of estrogens and failure to secrete such hormones as gonadoliberin (GnRH), the foliculothropic hormone (FSH) and the luteinizing hormone (LH) (5). Reduced production of estrogens contributes not only to impaired metabolism of the osseous tissue and development of osteoporosis, but also to the growing risk of neoplastic diseases. Abrupt loss of the bone mass is experienced during a few years following the menopause. Beyond the age of 65, the loss of the bone mass reaches approximately 25% of the maximum bone mass, while during the following years the decrease is remarkably lower (6).
Osteoporosis develops as the result of interaction between the environmental, hormonal and genetic factors which affect the bone mineral density and has a role in the risk of fracture. The environmental risk factors include the nutritional patterns: undernutrition, insufficient supply of calcium or vitamin D, lifestyle, including lack of physical activity, the use of substances, the number of pregnancies, the diseases suffered from, disorders of the bone marrow, any past inflammatory conditions or the drugs taken.
The bones change throughout the human life, where the process comprise bone resorption by osteoclasts and synthesis of the matrix by osteoblasts. It is estimated that the whole human skeleton in replaced every 10-25 years. This is necessary to adapt to the changing pressures on the skeleton throughout daily routines and to repair any microfractures or microdamages. Impaired balance between the osteoclasts and the osteoblasts may effect from hormonal changes or increased production of inflammatory cytokines or growth factors which results in reduced bone mass (osteoporosis) or the increased one (osteopetrosis). While osteopetrosis is a heterogenic group of rare hereditary diseases, osteoporosis, or the bone loss, is a common condition observed in numerous diseases, such as chronic infections, parodontosis, rheumatoid arthritis, leukaemia, postmenopausal osteoporosis or lytic bone metastases. Recently, it has become apparent that the immunological cells may have a role in bone physiology which gave birth to a new scientific discipline, named osteoimmunology.
Osteoclasts originate from the line of monocytes/macrophages are specialized cells responsible for bone decay (7). In normal bone remodeling the resorptive function of osteoclasts is coupled with formation of osteoblasts (8, 9). Bone remodeling is the basic process indispensable to preserve appropriate condition of the osseous tissue. The course of the process in physiological conditions in upon inflammation is presented in figure 1a, b.
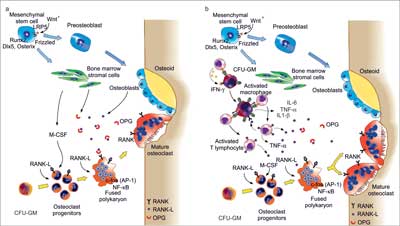
Fig. 1a, b. Bone remodeling under physiological conditions (homeostasis) (10) (a) and bone remodeling in chronic inflammation (10) (b).
a) Bone remodeling involves several types of cells: osteoblasts (OBs) which form the organic bone matrix; the bone marrow stromal cells and osteoclasts (OCs) resorbing a bone. Osteoblasts originate from the mesenchymal matrix cells. An important role in induction of osteoblastogenic transcription factors Runx2, Dlx5 and osterix is played by the pathways Wnt10 and LRP5. Osteoclasts are formed from the myeloid precursors influenced by RANK pathway and several transcription factors, e.g. NFκB i AP-1 (c-fos). RANK is activated by RANKL, member of TFN superfamily, which is a key osteoclastogenic cytokine, produced mainly by bone marrow cells and osteoblasts. RANKL activity is inhibited by osteoprotegerin, which is a soluble decoy receptor for RANKL. Bone homeostasis is regulated by the balanced resorptive activity of RANKL and the protective one of OPG.
b) Active lymphocytes T produce RANKL and its soluble form, therefore have a direct role in bone loss through induction and activation of OCs via RANKL. The quantity of RANKL exceeds the protective capacity of OPG. In addition, the proinflammatory cytokines, such as IL-1β, IL-6 and TNF-α, promote osteoclastogenesis and bone resorption, cooperating with RANKL. Gamma interferon (IFN-γ) promoted indirectly the bone decay through its capacity to trigger antigene presentation and activation of lymphocytes T through gene presenting cells
Numerous studies carried out throughout the recent years have significantly extended our knowledge of mechanisms underlying bone mass loss. Understanding of such processes is needed to design new therapeutic methods, which would slow down the disease progression in patients with bone mass loss. Discovery of three major particles controlling osteoblasts, therefore influencing osteoporosis and other bone conditions was a turning point to start a new era of medicine. A diagram showing their role in bone physiology is presented in figure 2 and the mechanism regulating osteoclastogenesis is illustrated in figure 3.
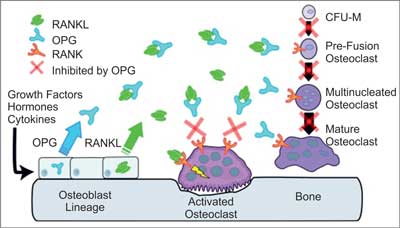
Fig. 2. The mechanism of OPG, RANKL and RANK activity (11). RANKL is expressed by osteoblasts, bone marrow stromal cells and other cells, affected by a number of proresorptive growth factors, hormones and cytokines. Osteoblasts and stromal cells express OPG which, binding with RANKL, inhibits its activity. The main binding complex is most probably a single OPG homodimer effecting at high affinity upon RANKL single homodimer. At the absence of OPG, RANKL activates its receptor RANK on osteoclasts and their precursors. Such activity of RANK-RANKL effects in:
– recruitment of preosteoclasts and their fusion into multinuclear osteoclasts,
– activation of osteoclasts,
– survival of osteoclasts.
Each of the responses may be inhibited by OPG
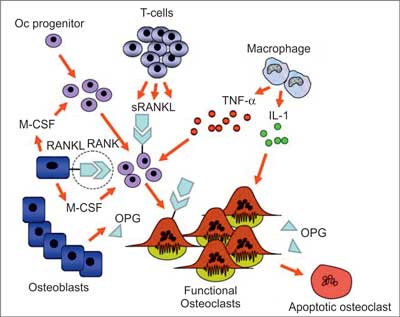
Fig. 3. Regulation of osteoclastogenesis (18). Differentiation of osteoclasts is regulated by osteoblasts. The process involves membrane-bound RANKL and soluble V-CSF. Expression of RANKL by osteoblasts is supported by the hormones, e.g. parathormone or cytokines, such as IL-6 and parathormone related protein (PTHrP) and reduced by estrogens. OPG is inhibited by glucocorticoids or PTH. (s)RANKL is also expressed by active lymphocytes T. TNF-α stimulates M-CSF and release of RANKL from osteoblasts which enhances osteoclastogenesis in inflammatory conditions. RANKL and IL-1 affect osteoclasts preventing their apoptosis. RANKL-RANK interaction (circled) may be therapeutically interfered by specific monoclonal antibodies of RANKL
Powyżej zamieściliśmy fragment artykułu, do którego możesz uzyskać pełny dostęp.
Mam kod dostępu
- Aby uzyskać płatny dostęp do pełnej treści powyższego artykułu albo wszystkich artykułów (w zależności od wybranej opcji), należy wprowadzić kod.
- Wprowadzając kod, akceptują Państwo treść Regulaminu oraz potwierdzają zapoznanie się z nim.
- Aby kupić kod proszę skorzystać z jednej z poniższych opcji.
Opcja #1
29 zł
Wybieram
- dostęp do tego artykułu
- dostęp na 7 dni
uzyskany kod musi być wprowadzony na stronie artykułu, do którego został wykupiony
Opcja #2
69 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 30 dni
- najpopularniejsza opcja
Opcja #3
129 zł
Wybieram
- dostęp do tego i pozostałych ponad 7000 artykułów
- dostęp na 90 dni
- oszczędzasz 78 zł
Piśmiennictwo
1. Kanis JA, Johnell O, Oden A et al.: Ten year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone 2002; 30: 251-258.
2. Kanis JA, Johnell O, Black DM et al.: Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: a reanalysis of the multiple outcomes of raloxifene evaluation trial. Bone 2003; 33: 293-300.
3. Straub RH, Cutolo M, Zietz B, Scholmerich J: The process of aging changes the interplay of the immune, endocrine and nervous systems. Mech Ageing Dev 2001; 122: 1591-1611.
4. Chahal HS, Drake WM: The endocrine system and ageing. J Pathol 2007; 211: 173-180.
5. Lukes A: Evolving issues in the clinical and managed care settings on the management of menopause following the women’s health initiative. Manag Care Pharm 2008; 14 (suppl. 3): 7-13.
6. Müller R: Long-term prediction of three-dimensional bone architecture in simulations of pre-, peri- and post-menopausal microstructural bone remodeling. Osteoporos Int 2005; 16 (suppl. 2): 25-35.
7. Sorensen MG, Henriksen K, Schaller S et al.: Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Miner Metab 2007; 25: 36-45.
8. Witten PE, Huysseune A: A comparative view on mechanisms and functions of skeletal remodeling in teleost fish, with special emphasis on osteoclasts and their function. Biol Rev Camb Philos Soc 2009; 84: 5-46.
9. Martin T, Gooi JH, Sims NA: Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr 2009; 19: 73-88.
10. Tilg H, Moschen AR, Kaser A et al.: Gut, inflammation and osteoporosis: basic and clinical concepts. Gut 2008; 57: 684-694.
11. Kearns AE, Khosla S, Kostenuik PJ: Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocrin Rev 2008; 29: 155-192.
12. So T, Lee SW, Croft M: Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int J Hematol 2006; 831: 1-11.
13. Kostenuik PJ, Shalhoub V: Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des 2001; 7: 613-635.
14. Schoppet M, Preissner KT, Hofbauer LC: RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol 2002; 22: 549-553.
15. Theill LE, Boyle WJ, Penninger JM: RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 2002; 20: 795-823.
16. Suda T, Kobayashi K, Jimi E et al.: The molecular basis of osteoclast differentiation and activation. Novartis Found Symp 2001; 232: 235-247.
17. Palmqvist P, Lundberg P, Persson E et al.: Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J Biol Chem 2006; 281: 2414-2429.
18. Romas E: Clinical applications of RANK – ligand inhibition. Int Med J 2009; 39: 110-116.
19. Robbins JA, Schott AM, Garnero P et al.: Risk factors for hip fracture in women with high BMD: EPIDOS study. Osteoporos Int 2005; 16: 149-154.
20. Blain H, Vuillemin A, Blain A et al.: Age-related femoral bone loss in men: evidence for hyperparathyroidism and insulin-like growth factor-1 deficiency. J Gerontol A Biol Sci Med Sci 2004; 59: 1285-1289.
21. Yarbrough DE, Barrett-Connor E, Morton DJ: Birth weight as a predictor of adult bone mass in postmenopausal women: the Rancho Bemardo Study. Osteoporos Int 2000; 11: 626-630.
22. Antoniades L, MacGregor AJ, Andrew T, Spector TD: Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology 2003; 42: 791-796.
23. Boroń D, Kotrych D, Bartkowiak-Wieczorek J et al.: Polymorphisms of OPG and their relation to the mineral density of bones in pre- and postmenopausal women. Int Immunopharmacol 2015; 28: 477-486.
24. Boroń D, Plewka A, Jędrusik P: Polimorfizm 1181G/C genu OPG i jego związek z osteoporozą u kobiet po menopauzie. Ann Acad Med Siles 2014; 68: 407-414.
25. Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF: Polymorphisms in the osteoprotegerin gene are associated with osteoporotic fractures. J Bone Mineral Res 2002; 17: 1245-1255.
26. Ohmori H, Makita Y, Funamizu M et al.: Linkage and association analyses of the osteoprotegerin gene locus with human osteoporosis. J Hum Genet 2002; 47: 400-406.
27. Vidal C, Brincat M, Xuereb Anastasi A: TNFRSF11B gene variants and bone mineral density in postmenopausal women in Malta. Maturitas 2006; 53: 386-395.
28. Arnold A, Horst SA, Gardella TJ et al.: Mutation of the signal peptide encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest 1990; 86: 1084-1087.
29. Hofbauer LC, Khosla S, Dunstan CR et al.: The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 2000; 15: 2-12.
30. Hofbauer LC, Khosla S, Dunstan CR et al.: Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 1999; 140: 4367-4370.
31. Lochlin RM, Khosla S, Turner RT, Riggs BL: Mediators of the bisphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 2003; 89: 180-190.
32. Hofbauer LC, Gori F, Riggs BL et al.: Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells. Endocrinology 1999; 140: 4382-4389.
33. Kong YY, Feige U, Sarosi I et al.: Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through Osteoprotegerin ligand. Nature 1999; 402: 304-309.
34. Takayanagi H, Iizuka H, Juji T et al.: Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 2000; 43: 259-269.
35. Boroń D, Plewka A, Jędrusik P: Polimorfizm 575C/T genu RANK i jego związek z osteoporozą u kobiet po menopauzie. Ann Acad Med Siles 2014; 68: 192-199.
36. Boroń D, Plewka A, Jędrusik P: Polimorfizm -643C/T genu RANKL i jego związek z osteoporozą u kobiet po menopauzie. Ann Acad Med Siles 2014; 68: 415-423.
37. Lu Y, Liu P, Recker RR et al.: TNFRSF11A and TNFSF11 are associated with age at menarche and natural menopause in white women. Menopause 2010; 17: 1048-1054.
38. Gordon CM, DePeter KC, Feldman HA et al.: Prevalence of vitamin D defi ciency among healthy adolescents. Arch Pediatr Adolesc Med 2004; 158: 531-537.
39. Holick MF: High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81: 353-373.
40. Lips P, Hosking D, Lippuner K et al.: The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 2006; 260: 245-254.
41. Bischoff-Ferrari HA, Dietrich T, Orav E, Dawson-Hughes B: Positive association between 25(OH)D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 2004; 116: 634-639.
42. Cashman KD: Vitamin D in childhood and adolescence. Postgrad Med J 2007; 83: 230-235.
43. Holick MF: Vitamin D deficiency. N Engl J Med 2007; 357: 266-281.
44. Springer JE, Cole DE, Rubin LA et al.: Vitamin D receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology 2000; 118: 145-151.
45. Chen HY, Chen WC, Hsu CD et al.: Relation of vitamin D receptor FokI start codon polymorphism to bone mineral density and occurrence of osteoporosis in postmenopausal women in Taiwan. Acta Obstet Gynecol Scand 2002; 81: 93-98.
46. Langdahl BL, Gravhold CH, Brixen K, Eriksen EF: Polymorphism in the vitamin D receptor gene and bone mass, bone turnover and osteoporotic fractures. Eur J Clin Invest 2000; 30: 608-617.
47. Boroń D, Kamiński A, Kotrych D et al.: Polymorphism of vitamin D3 receptor and its relation to mineral bone density in perimenopausal women. Osteoporos Int 2015; 26: 1045-1052.
48. Horst-Sikorska W, Wawrzyniak A, Celczyńska-Bajew L et al.: Polymorphism of VDR gene – the most effective molecular marker of osteoporotic bone fractures risk within postmenopausal women from Wielkopolska region of Poland. Pol J Endocrinol 2005; 3: 233-239.
49. Garnero P, Munoz F, Borel O et al.: Vitamin D receptor gene polymorphisms are associated with the risk of fractures in postmenopausal women, independently of bone mineral density. J Clin Endocrinol Metab 2005; 90: 4829-4835.
50. Moffett SP, Zmuda JM, Cauley JA et al.: Association of the VDR translation start site polymorphism and fracture risk in older women. J Bone Miner Res 2007; 22: 730-736.
51. Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF: Polymorphisms in the transforming growth factor beta 1 gene and osteoporosis. Bone 2003; 32: 297-310.
52. Alvarez-Hernandez D, Navez M, Diaz-Lopez JB, Gomez C: Influence of polymorphisms in VDR and COL1A1 genes on the risk of osteoporotic fractures in aged men. Kidney Int 2003; suppl. (85): S14-18.
53. Kearns AE, Khosla S, Kostenuik PJ: Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 2008; 29: 155-192.
54. Koshy PJ, Henderson N, Logan C et al.: Interleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proin?ammatory cytokines. Ann Rheum Dis 2002; 61: 704-713.
55. Agarwal S, Misra R, Aggarwal A: Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol 2008; 35: 515-519.
56. Boroń D, Seremak-Mrozikiewicz A, Kotrych D et al.: Polymorphism of interleukin-17 and its relation to mineral density of bones in perimenopausal women. Eur J Med Res 2014; 19(69): 1-7.
57. Miossec P: Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum 2007; 56: 2111-2115.
58. Bayer C, Schett G: Novel targets in bone and cartilage. Best Pract Res Clin Rheumatol 2010; 24: 489-496.
59. Zhao B, Takami M, Yamada A et al.: Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 2009; 15: 1066-1071.
60. Yago T, Nanke Y, Ichikawa N et al.: IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-α antibody: a novel mechanism of osteoclastogenesis by IL-17. J Cell Biochem 2009; 108: 947-955.
61. Poulos SP, Hausman DB, Hausman GJ: The development and endocrine functions of adipose tissue. Mol Cell Endocrinol 2010; 323: 20-34.
62. Xie H, Xie PL, Wu XP et al.: Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc Res 2011; 92: 296-306.
63. De Souza Batista CM, Yang RZ, Lee MJ et al.: Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007; 56: 1655-1661.
64. Thommesen L, Stunes AK, Monjo M et al.: Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem 2006; 99: 824-834.
65. Xie H, Tang SY, Luo XH et al.: Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int 2007; 80: 201-210.
66. Guo LJ, Jiang TJ, Liao L et al.: Relationship between serum omentin-1 level and bone mineral density in girls with anorexia nervosa. J Endocrinol Invest 2013; 36: 190-194.
67. Tohidi M, Akbarzadeh S, Larijani B et al.: Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women. Bone 2012; 51: 876-881
68. Xie H, Xie PL, Luo XH et al.: Omentin-1 exerts bone-sparing effect in ovariectomized mice. Osteoporos Int 2012; 23: 1425-1436.
69. Boroń D, Czerny B, Bartkowiak-Wieczorek J et al.: Omentin polymorphism and its relations to bone mineral density in women. Arch Med Res 2015; 46: 173-180.



