Zuzanna Gorski, *Lidia Zawadzka-Głos
A review of endoscopic sinus surgery in the management of chronic rhinosinusitis and nasal polyposis in pediatric cystic fibrosis patients
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Lidia Zawadzka-Głos, MD, PhD
Summary
Introduction. Cystic fibrosis (CF) is an autosomal recessive disease affecting the epithelial lining of the respiratory tract and exocrine glands (1-5). Many children suffering from CF are often diagnosed and treated for various co-morbidities, including chronic rhinosinusitis (CRS) and nasal polyposis (NP) (3, 4, 6, 7), which will remain the focus of this article.
Aim. The aim of this study was to examine the characteristic of patients with cystic fibrosis (CF) admitted to the Pediatric Otolaryngology Department due to coexisting chronic rhinosinusitis (CRS) or nasal polyposis (NP). The study focused on the demographics, symptoms and management of children with CF with coexisting CRS and/or NP. The data was then compared to the results that had been presented in the literature.
Material and methods. A retrospective study of 26 pediatric patients previously diagnosed with CF that were admitted to the Department of Pediatric Otolaryngology of the Medical University of Warsaw between 2010 and 2015 was conducted. Patients’ medical histories were carefully reviewed. Data on patients’ age, gender, symptoms and CF comorbidities were collected. The number and type of procedures performed on each patient were documented. Further assessment of the localization of polyps was performed in all NP-positive patients.
Results. The study included 26 patients (15 males and 11 females). Mean age was 9 years. CRS and NP was present in 100% and 88.5% of the patients, respectively. 23 children underwent a total of 35 sinus surgeries due to CRS and/or NP. 6 patients required one or more revision surgeries, with a total revision rate of 54.1%. Adenoidectomy (AT) and/or adenotonsillectomy (ATT) was performed in 10 patients. 5 children were disqualified from the surgery, due to various reasons. The most common localization of NP was maxillary sinus, followed by ethmoid sinus, sphenoid sinus, frontal sinus, and nasal cavity.
Conclusions. Due to a wide range of clinical findings in many organs and high variability of symptoms in individual cases, there is currently no standardized treatment regimen for pediatric CF patients with CRS or NP. Early intervention and a multidisciplinary approach are highly recommended, due to a positive correlation between the increase in patient’s age and the number of admissions and reoperations. Endoscopic sinus surgery should be considered in CF patients with refractory, chronic or severe acute CRS or NP.
INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive disease, in which the function of the cystic fibrosis transport regulator (CFTR) gene located on chromosome 7, responsible for chloride ion transport, is compromised. This results in a dysfunction of the epithelial cells lining the respiratory tract, as well as of the exocrine glands (1-5). The organs that are most severely affected are airways, pancreatic ductal system, hepatobiliary system, and male ductus deferens (1, 3). Clinical symptoms may occur as early as after birth or in early childhood, and commonly include pancreatic insufficiency, seen in 90% of infants with cystic fibrosis, vitamin malabsorption, impaired growth and development, and respiratory manifestations, such as recurrent infections, sinusitis, bronchiectasis, and sinonasal polyposis (3, 4, 6, 7). It is important to note that respiratory tract and sinonasal complications due to CFTR malfunction are the most common cause of premature death in CF patients (3). The respiratory symptoms and complications appear to be caused mainly by defective mucociliary drainage, followed by subsequent bacterial colonization – with most common pathogens being Staphylococcus aureus and Pseudomonas aeruginosa (1, 2, 6, 8). The direct pathogenic action of the bacteria, along with the consequent local inflammation and chronic nasal obstruction, play a central role in airway destruction, respiratory failure, and early death in CF patients (1, 3, 8). Two comorbidities that are commonly encountered in CF patients include chronic rhinosinusitis (CRS) and nasal polyposis (NP), which were the focus of the study.
Due to the accumulation of a thick mucus within the sinonasal area, a great number of CF patients consequently develop CRS. CRS is defined as a sinus infection lasting for more than 12 weeks with low-grade signs and symptoms (2, 5, 9-11). The main reasons for the development of CRS are decreased mucociliary clearance, infections, coexisting allergies, edema of the mucosa, and, less commonly, anatomic abnormalities of the sinonasal area (2, 12).
Due to the early onset and subsequent adaptation of the patient to the symptoms of CRS, no more than 10% of the CF patients report that they perceive their symptoms as severe (1, 13, 14). The most commonly reported symptoms include: nasal obstruction and discharge, which lead to mouth breathing, post-nasal drip, headache, and localized facial pain or feeling of pressure (1, 9, 12, 15). Adenoid hypertrophy or inflammation may also be present (14). Although a high number of CRS cases in CF patients are of idiopathic origin, other noteworthy non-CF causative factors of CRS include allergy, aspirin sensitivity, and immunodeficiency (13).
Nasal polyposis (NP) can have multiple causes, however, chronic neutrophil-dominant inflammation, commonly present in CF patients, is believed to be one of the major ones (1, 3, 7, 16). The prevalence of NP in CF patients has been increasing over the years (1). Primary appearance of NP in children may be seen in children as young as kindergartners (1, 14). Nasal polyps are present in approximately 86% of CF patients, however, the prevalence has been shown to be highly variable between individual study populations (2, 6, 14). During the first appearance of nasal polyps, it is recommended to refer the patient to an ENT surgeon for further examination (10). If not previously diagnosed with CF, children presenting with nasal polyps should always undergo further testing for CF (10).
Endoscopic examination, as well as radiological imaging, play a central role in diagnostics and staging of CRS and NP in CF patients (1). Although endoscopic examination in CF patients is consistently abnormal, it is essential in determining the presence of nasal polyps (14). Several radiologic imaging techniques for diagnosing CRS and NP have been discussed in the literature, including spiral multislice CT, digital volume CT, and MRI; MRI is the preferred modality in children (1, 2). MRI offers a sensitive and superior visualization of the mucosa, polyps and other intracranial soft tissue abnormalities (1, 17).
Up to date, there has been no standardized treatment for NP in CF patients due to the high variability of the stage of the disease between individual patients and the lack of strong evidence supporting a treatment that would be successful in a large percentage of the patients (1, 2, 18). A recent review conducted by Mainz and Koitschev (1) summarized several conservative treatment options, including topical application of nasal sprays and drops, lavages and inhalations, nasal saline irrigations, topical decongestants, steroids, antibiotics, immunomodulators, antimycotics, mucolytics, bacterial lysates, antihistamines and monoclonal antibodies. No specific topical or systemic conservative therapies were proven to have significant curative outcomes on NP in CF. Therefore, surgical intervention is the treatment of choice once conservative measures are no longer beneficial for an individual, or in instances of chronic and recurrent disease (1, 4, 13, 18).
Surgical intervention is always preceded by imaging of the nasal cavity and paranasal sinuses, in order to confirm the anatomy and localization of the lesions and to design a specific surgical approach for the removal (1). In addition, the severity of sinonasal symptoms and the grade of the coexisting pulmonary dysfunction are the factors favoring surgery (1). Presently, the most widely used surgical intervention is functional endoscopic sinus surgery (FESS), which is minimally invasive and is associated with enhanced recovery from sinus-related symptoms and an improved subsequent quality of life (18). Two definitive indications for conducting FESS are NP and CRS unresponsive to conservative therapy (19, 20). FESS in children is performed under general anesthesia, whereas in adults, it may be performed under sedation with topical and local anesthesia (21). FESS procedures are highly personalized and dependent on the severity and extent of the changes (20, 21). Many types of FESS may be performed, from the isolated infundibulotomy or the resection of the uncinate process, to a complete sphenoeithmoidectomy, which is, however, very rarely performed (19, 21). Contraindications to FESS include the presence of obstructions or stenoses, which may severely reduce the operative field, as well as any signs of meningitis, other intracranial lesions, severe sinus infections, osteomyelitis of the frontal bone, or orbital cellulitis with visual field defects (20, 21). The aim of sinus surgery in CF patients consisting of enlarging the sinus ostia is to allow improved drainage of the sinuses, eliminate mucosal inflammatory changes such as NP, remove any infected tissue, and permit proper healing and epithelialization of the surgically enlarged ostiomeatal complex (14, 22). The procedure is generally focused on the anterior ethmoidal cells and ostiomeatal complex, since these areas are highly associated with the proper functioning of the remaining sinuses (20). Other more rarely performed extensive surgeries include ethmoidectomy, septoplasty, or the now historic Caldwell-Luc procedure (2, 21). In severe and complicated cases of NP presenting with extensive obstruction, more aggressive and extensive surgical approaches are considered (11, 12).
MATERIAL AND METHODS
A retrospective study on 26 pediatric patients previously diagnosed with CF who had been admitted to the Pediatric Otolaryngology Department at the Medical University of Warsaw between the years 2010 and 2015 was conducted. Patients’ medical histories were carefully reviewed. Symptoms of sinonasal disease were gathered from the interviews with the patient and/or parents, depending on the patient’s age. All available pre-operative endoscopic examinations and imaging studies were analyzed. All 26 patients were admitted to the Department due to clinical symptoms of a various degree. The data on patients’ age, gender and comorbidities were collected. The number and type of procedures performed on each patient were documented. Further assessment of the exact localization of polyps in all NP positive patients was performed.
RESULTS
The mean age of the patients was 9 years (with the youngest patient being 3 and the oldest 16 years). There were 11 male patients (42.3%) and 15 female patients (57.7%). The patients were divided into groups according to age, with 7 patients (26.9%) aged less than 6, 12 patients (46.2%) aged 7 to 12 years, and 7 patients (26.9%) aged 13 to 18 years. Patients were admitted to our Department after the referral from primary specialist care or a different hospital department. With a total number of 45 admissions for 26 patients, 17 patients (65.4%) were admitted once, 4 patients (15.5%) were admitted twice, 3 patients (11.5%) were admitted 3 times, 1 patient (3.8%) was admitted 5 times, and 1 patient (3.8%) was admitted 6 times. An increase in the frequency of admissions and the number of surgeries correlated with an increase in patient’s age, as represented in figures 1 and 2.
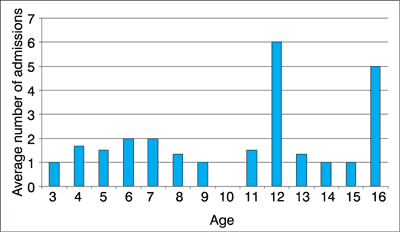
Fig. 1. Average number of admissions by age of the patient
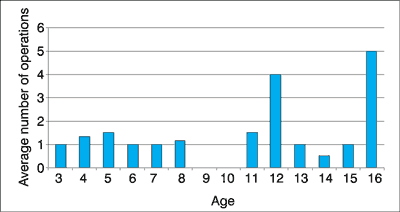
Fig. 2. Average number of operations by age
Symptoms at the time of admission included nasal congestion and secretion, nasal obstruction, post-nasal drip, headache, and adenoid hypertrophy. All the patients were positive for CRS, whereas 23 out of 26 patients (88.5%) additionally presented with NP. Clinical examination and studies revealed deviated nasal septum in 7 patients, secretory otitis media in 2 patients, allergies in 2 patients, and von Willebrand disease type 1 in 1 patient. 2 out of 7 patients (28.6%) with a deviated nasal septum underwent septoplasty, whereas both cases (100%) of secretory otitis media were treated with tympanocentesis. Both patients (100%) with coexisting allergies underwent maxillary antrostomy. A total of 54 procedures were performed. The most common procedure was FESS, which was performed 35 times (64.8%), followed by adenoidectomy (AT), performed 11 times (20.3%), maxillary antrostomy, performed 3 times (5.6%), tympanocentesis, performed 2 times (3.7%), septoplasty, performed 2 times (3.7%), and sinus puncture, performed once (1.9%). 35 total of FESS procedures were performed on 23 children diagnosed with NP. Among these 23 patients, 17 patients (73.9%) underwent only 1 surgery, whereas 3 patients underwent 2 surgeries, 1 underwent 3 surgeries, 1 underwent 4 surgeries, and 1 underwent 5 surgeries. The overall revision rate for FESS was 51.4%. 84.3% of the polyps were located in the paranasal sinuses, 13.3% in the nasal cavity, and 2.4% were antrochoanal polyps. In the paranasal sinuses, the most commonly affected sinus was the maxillary sinus (31.4%), followed by ethmoid sinus (25.7%), sphenoid sinus (22.8%), and frontal sinus (20.0%). A total of 11 adenoidectomies (AT) and/or adenotonsillectomies (ATT) were performed on 10 patients, and one patient required a revision AT procedure. 3 patients (30%) underwent AT/ATT prior to sinus surgery, compared to 7 patients (70%) in which AT/ATT was performed during the FESS. The revision procedure was undertaken following a primary peri-operative AT.
DISCUSSION
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the CFTR gene (1-5). Though CF may manifest through a wide range of clinical findings many organs, the aim of this study was to focus on patients admitted due to CRS or NP, who were in need of otolaryngologic consultation or treatment. There is no standard treatment algorithm, as the severity of the disease is different for every patient, therefore, the treatment of each patient is considered individually (1, 2, 18).
Several diagnostic imaging techniques for CRS and NP in CF patients are used, the most important being endoscopy, CT, and MRI. Some authors have suggested that CT study is unable to reliably assess the severity of disease; therefore, the decision on surgical treatment should not be based solely on the results of the imaging studies (14). Patient’s medical history, their complaints, and the quality of life are also important factors that must be taken into account, considering the high individual variability of the severity of the disease in CF patients (1, 18). Only upon gathering both subjective and objective data can a proper treatment modality be considered (1). Prompt involvement of an interdisciplinary team including otolaryngologists, microbiologists and CF specialists is the key to early diagnosis and effective treatment (16).
Due to a high number of causative factors, CRS is extremely common in CF patients, with prevalence reaching close to 100% in some study groups (2, 6, 16). All 26 pediatric patients (100%) admitted to our Department suffered from CRS. Allergy, among others, has been found to be a contributing factor to the development of CRS (13). 2 patients in our study were diagnosed with allergy, one of which required 3 revision surgeries; it can be hypothesized that this was due to an increased allergy-induced recurrence of sinonasal symptoms. A study on 126 CF patients aged from 4 to 28 years with coexisting CRS was conducted by Babinski and Trawinska-Bartnicka (5). CRS among these patients was classified into one of the four types (listed from the highest to the lowest prevalence): infectious chronic, infectious acute, allergic chronic and non-allergic with eosinophilia (5). Nasal polyps were present in 18.3% of patients, and were found to correlate with the type of mutation in the CFTR gene (5). The average age at the diagnosis of CRS ranged from 2.2 to 13.5 years (5).
Previously, 74% of CF patients were found to have a bilateral medial displacement of the lateral nasal wall and bilateral uncinate process demineralization (2, 14), however, only 7 out of 26 patients (26.9%) in our study presented with a deviated nasal septum and no cases of uncinate process demineralization were observed. Septoplasty was performed in 2 out of the 7 patients (28.6%) who presented with severe septal deviation and required surgical intervention. Overall, septoplasty was performed in 2 out of 23 patients (8.7%) that underwent FESS. A study of 77 patients that received endoscopic sinus surgery completed by Stankiewicz showed a slightly higher rate of septoplasty (15.6%) among their patients (23).
The frequency of NP in CF patients has high variability among studies and is reported to be about 86% (6). Despite the obstructive nature of NP, a recent review by Feuillet-Fieux et al. (25) concluded that NP is not clearly associated with a poor outcome in CF, however, the patients experience a higher incidence of acute exacerbations and hospitalizations (1, 24, 25). In pediatric CF population, more NP cases are observed in older children (1). Nonetheless, primary appearances of NP in children may be seen in patients as young as kindergartners (1, 5, 14). Increasing prevalence of NP occur in adolescence, ranging from 6% up to 44.8% of the CF patients (1, 5, 26). In our study, 23 out of all 26 CF patients were positive for NP, with overall prevalence rate of 88.5%, which is comparable to 86%, seen in previous publications (6). The youngest and oldest patients presenting with NP were 4 and 16 years, respectively. In a study conducted by Schraven et al. (16) information on 81 CF patients was collected over a 10-year period. Sinus surgery was performed on 19.6% (16 out of 81) CF patients with chronic polypoid sinusitis (CPS). Prevalence of CPS in CF patients aged less than 6 years, 7 to 12 years, and adolescents (13 to 18 years old), was calculated, and accounted 19, 38 and 45%, respectively (16). When categorized into the same age groups, the prevalence of NP in our patients was 26.1, 47.8 and 26.1%, respectively, with children aged 7 to 12 having the highest prevalence of NP. This, however, could be due to the high number of children in this age group, being close to half of the all patients included in our study.
After a careful review of patient records and operative notes, the specific location of polyps in children with NP was documented and divided into groups by location: polyps in the paranasal sinuses, (further divided into maxillary, sphenoid, ethmoid and frontal sinuses), in the nasal cavity, and antrochoanal polyps. 84.3% of polyps were located in the paranasal sinuses, 13.3% in the nasal cavity, and 2.4% were antrochoanal polyps. In the paranasal sinuses, the most commonly affected sinuses were the maxillary sinus (31.4%), ethmoid sinus (25.7%), sphenoid sinus (22.8%), and frontal sinus (20.0%). The number of surgeries that individual patient had undergone depended on the age of the patient and the location of the polyp. However, independently of patient’s age or number of operations, paranasal sinuses were consistently the most common area in which polyps were found, followed by the nasal cavity and antrochoanal polyps. Furthermore, in the paranasal sinuses, polyps were most frequently observed in the maxillary sinus, followed by ethmoid, sphenoid and frontal sinus, in all the age groups of the patients.
There has been limited data in previous literature pertaining to the location of polyps in CF patients that undergo endoscopic sinus surgery. Stankiewicz (23) reported the effects of endoscopic sinus surgery performed on 83 patients, 5 of which were CF patients (28 male, 55 female, aged from 1 to 18 years). Pansinusitis was present in 51 patients (61.5%) (bilateral in 40 patients and unilateral in 11), and the disease was present in the maxillary sinus, ethmoid sinus, sphenoid sinus, fontal sinus in 8, 8, 6, and 4 patients, respectively, which complies to the location of polyps we have observed in our study.
Antrochoanal polyps are relatively rare and therefore, they will be treated on separately. Arising from the maxillary antrum, antrochoanal polyps (ACPs) extend through the ostium and protrude posteriorly to the choana and nasopharynx (27). These polyps are usually present unilaterally and occur in younger patients than other nasal polyps, which tend to manifest bilaterally and occur in older patients (28). ACPs are one of the most common nasal polyps seen in non-CF children and young adults. An effective treatment for ACP is endoscopic wide middle meatalantrostomy (27, 29). Lee and Huang (29) described 26 pediatric patients who underwent sinus surgery for ACPs, 12 of which (46%) were male and 14 (56%) were female. The average age was 8.7 years (from 5 to 15 years). Depending on the location of the ACP, 13 patients were operated with a transnasal endoscopic approach and 13 were operated with a combined endoscopic and transcanine approach, with success rates reaching 76.9% and 100%, respectively (29). ACPs were present in 2 out of 26 CF patients (7.7%) in our study. The average age in our study was 6.5 years, which is slightly lower than 8.7 years described by Lee and Huang (29). Both our patients did not require additional revision surgeries, therefore, the success rate was 100%. To maximize the effectiveness of surgical treatment for both ACPs and NP, it is crucial to properly recognize and remove both the origin and the main bulk of the polyp (29).
In 2007, the British Society for Allergy and Clinical Immunology (BSACI) guidelines for the management of rhinosinusitis and nasal polyposis were published (10). Surgery was recommended to be reserved for patients with severe acute or persistent chronic symptoms, or those resistant to previous medical therapy (10). 20-25% of the CF patients will require surgery due to CRS after medical management has failed (2). It is believed that this value will grow due to an increasing survival rate for CF over the years, the median life expectancy being 40 years of age now (2, 3, 16). The mean age at the pediatric FESS surgery in CF patients suffering from CRS varies between centers, ranging from 2.6 to 14.5 years (15, 18). In our study group, the mean age was 9 years. There are concerns that FESS on pediatric patients may have influence on facial growth and symmetry, however, there has been a substantial lack of confirming evidence (30).
The use of endoscopy in postoperative management follow-up helps in the early recognition of recurrence, thanks to the high quality of illumination and visualization of the operated area (20). Previous publications have reported 50-100% recurrence rates of nasal polyps in CF patients post FESS, with 83% of children requiring at least 1 revision operation (18). Among the patients in our study, the overall revision rate for FESS was 51.4%.
The need for reoperation is predominantly due to the chronic nature of CF (2). 44% of re-operated children require revision surgery due to poor post-operative cleansing of the nasal cavity (23). The reoperations of recurrent NP in CF patients are mainly due to extensively thickened mucus with subsequent crusting, chronic bacterial infections, and impeded or defective healing of the mucosa post-operatively (12, 13, 17). These factors collectively may stimulate the growth of granulation tissue, clinically resembling recurrent NP (17). A median time of 4 years was observed between sinus surgeries for CRS in CF patients in a small study conducted by Yung et al. (31).
A systematic literature review conducted by Vlastarakos et al. (18) included 13 research papers, with a total of 1301 pediatric patients, who underwent FESS due to CRS. Although between 71% and 100% of children had positive outcome of the procedure, the authors concluded that pediatric FESS shows a lower therapeutic benefit in the presence of an underlying systemic disease, such as CF (13). 8 out of the 13 research groups concerned pediatric CF patients. The quality of life and symptoms, such as nasal congestion and discharge, improved in CF patients, however, the improvement lasted for a shorter period and the recurrence rate was higher than in otherwise healthy children (18). Endoscopic sinus surgery has not been shown to significantly improve pulmonary function tests in the long term, measured by forced vital capacity (FVC) and forced expiratory volume (FEV1), nor does it affect microbial colonization in CF patients (4, 6, 30). There is high variability in long-term outcomes of sinus surgery in CF patients, suggesting that more data is needed to aid in evaluation and selection of the patients who would benefit from this procedure (14).
The overall incidence of major complications after pediatric FESS surgery is 0.6% (18). This includes bleeding, CSF leak, and meningitis (none was reported to be fatal or to have irreversible consequences) (18). Reported minor complications, with a incidence lower than 2%, included minor bleeding, lamina papyracea breach, periorbital ecchymosis or surgical emphysema, and meatal scarring/adhesions/synechiae (18). Caution should be taken to avoid nasolacrimal duct injury as well (23).
Comparison of patient data, including gender, age, number of total surgeries, number of sinus surgeries, and average age at time of operation, is presented in table 1. It is comparable to patient demographics described in previous studies (4, 6, 7, 12, 30).
Tab. 1. Comparison of patient demographics, number of total operations, number of sinus operations and average number of operations per patient between six study groups
| Studies | # of CF patients | Males | Females | Avg age (range) | # of total operations | # of sinus operations | Average operations per patient |
| Current Study | 26 | 11 (42.3%) | 15 (57.7%) | 9 (3-16) | 54 | 35 | 2.07 |
| Osborn 2011 | 41 | 24 (58.5%) | 17 (41.5%) | 11.9 (5-18) | 91 | NA | 2 |
| Kovell 2011 | 62 | 34 (54.9%) | 28 (45.1%) | 11 (1-21) | NA | 21 | NA |
| Keck 2007 | 26 | 9 (34.6%) | 17 (65.4%) | 14.6 (3-33) | 42 | NA | 1.6 |
| Knipping 2007 | 26 | 17 (65.4%) | 9 (34.6%) | NA (3-16) | NA | NA | NA |
| Rosbe 2001 | 66 | 34 (51.5%) | 32 (48.5%) | 17 (NA) | 112 | NA | 1.8 |
NA= Not Available
Sinusitis may be accompanied by purulent adenoiditis or adenoid hypertrophy, which may further exacerbate symptoms through nasal obstruction and accumulation of nasal secretion (23). Performing an adenoidectomy (AT) or adenotonsillectomy (ATT) simultaneously with sinus surgery has been described to increase the percentage of a successful procedures in patients with severe disease (23). The decision to perform AT or ATT prior to surgery is highly individual and based on the surgeon’s opinion and experience, but may be recommended in children with recurrent infections and minimally positive CT scans (23). AT/ATT was performed in 43.5% of our patients (10 of 23) who underwent FESS, which is slightly higher than the rate of AT performed in patients undergoing sinus surgery in the study by Stankiewicz (23; 26% of patients, 20 of 77). The majority of our patients (70%) underwent AT/ATT during FESS, whereas the remaining 30% received AT/ATT prior to FESS. 1 patient required a revision AT, which was performed after FESS.
CONCLUSIONS
Due to a wide range of clinical findings in many organs and high variability of symptoms in individual cases, there is currently no standardized treatment regimen for pediatric CF patients with NP or CRS (2, 6, 16). Early intervention and a multidisciplinary approach are highly recommended (16), due to a positive correlation between an increase in patient’s age and the number of admissions and reoperations. After the initial diagnosis of nasal polyps, it is recommended to refer the patient to an ENT surgeon for further examination (10). If not previously diagnosed with CF, children presenting with nasal polyps should always undergo further testing for CF (10). FESS is recommended to be considered in CF patients with refractory, chronic, or severe acute CRS and NP (10). It is considered to be one of the most successful treatment modalities for these patients. Postoperative complication rate is low, and most of them are minor and non-life threatening (18, 23). CRS is frequently associated with purulent adenoiditis or adenoid hypertrophy, thus requiring the surgeon to perform AT and/or ATT prior to or during FESS. Due to the chronicity of CF, it is important to keep in mind that CF patients with CRS and NP may require revision surgery.
Piśmiennictwo
1. Mainz JG, Koitschev A: Pathogenesis and management of nasal polyposis in cystic fibrosis. Curr Allergy Asthma Rep 2012 Apr; 12(2): 163-174. 2. Oomen KP, April MM: Sinonasal manifestations in cystic fibrosis. Int J Otolaryngol 2012; 2012: 789572. 3. Flume PA, Van Devanter DR: State of progress in treating cystic fibrosis respiratory disease. BMC Med 2012 Aug 10; 10: 88. 4. Kovell LC, Wang J, Ishman SL et al.: Cystic fibrosis and sinusitis in children: outcomes and socioeconomic status. Otolaryngol Head Neck Surg 2011 Jul; 145(1): 146-153. 5. Babinski D, Trawinska-Bartnicka M: Rhinosinusitis in cystic fibrosis: not a simple story. Int J Pediatr Otorhinolaryngol 2008 May; 72(5): 619-624. 6. Osborn AJ, Leung R, Ratjen F, James AL: Effect of endoscopic sinus surgery on pulmonary function and microbial pathogens in a pediatric population with cystic fibrosis. Arch Otolaryngol Head Neck Surg 2011 Jun; 137(6): 542-547. 7. Knipping S, Holzhausen HJ, Riederer A, Bloching M: Cystic fibrosis: ultrastructural changes of nasal mucosa. Eur Arch Otorhinolaryngol 2007 Dec; 264(12): 1413-1418. 8. Mainz JG, Naehrlich L, Schien M et al.: Concordant genotype of upper and lower airways P. aeruginosa and S. aureus isolates in cystic fibrosis. Thorax 2009 Jun; 64(6): 535-540. 9. Fokkens WJ, Lund VJ, Mullol J et al.: EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012 Mar; 50(1): 1-12. 10. Scadding GK Durham SR, Mirakian R et al.: BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy 2008 Feb; 38(2): 260-275. 11. Clement PA, Bluestone CD, Gordts F et al.: Management of rhinosinusitis in children: consensus meeting, Brussels, Belgium, September 13, 1996. Arch Otolaryngol Head Neck Surg 1998 Jan; 124(1): 31-34. 12. Keck T, Rozsasi A: Medium-term symptom outcomes after paranasal sinus surgery in children and young adults with cystic fibrosis. Laryngoscope 2007; 117(3): 475-479. 13. Ryan MW, Brooks EG: Rhinosinusitis and comorbidities. Curr Allergy Asthma Rep 2010 May; 10(3): 188-193. 14. Robertson JM, Friedman EM, Rubin BK: Nasal and sinus disease in cystic fibrosis. Paediatr Respir Rev 2008 Sep; 9(3): 213-219. 15. Makary CA, Ramadan HH: The role of sinus surgery in children. Laryngoscope 2013 Jun; 123(6): 1348-1352. 16. Schraven SP, Wehrmann M, Wagner W et al.: Prevalence and histopathology of chronic polypoid sinusitis in pediatric patients with cystic fibrosis. J Cyst Fibros 2011 May; 10(3): 181-186. 17. Eggesbø HB, Dølvik S, Stiris M et al.: Complementary role of MR imaging of ethmomaxillary sinus disease depicted at CT in cystic fibrosis. Acta Radiol 2001 Mar; 42(2): 144-150. 18. Vlastarakos PV, Fetta M, Segas JV et al.: Functional endoscopic sinus surgery improves sinus-related symptoms and quality of life in children with chronic rhinosinusitis: a systematic analysis and meta-analysis of published interventional studies. Clin Pediatr (Phila) 2013 Dec; 52(12): 1091-1097. 19. Kennedy DW: Functional endoscopic sinus surgery: technique. Arch Otolaryngol 1985. 111(10): 643-649. 20. Shah NJ: Functional Endoscopic Sinus Surgery (1999). 21. Stammberger H, Posawetz W: Functional endoscopic sinus surgery. Eur Arch Otorhinolaryngol 1990; 247(2): 63-76. 22. Maran A: Endoscopic sinus surgery. Eur Arch Otorhinolaryngol 1994; 251(6): 309-318. 23. Stankiewicz JA: Pediatric endoscopic nasal and sinus surgery. Otolaryngol Head Neck Surg 1995; 113(3): 204-210. 24. Henriksson G, Westrin KM, Karpati F et al.: Nasal polyps in cystic fibrosis: clinical endoscopic study with nasal lavage fluid analysis. Chest 2002 Jan; 121(1): 40-47. 25. Feuillet-Fieux MN, Lenoir G, Sermet I et al.: Nasal polyposis and cystic fibrosis (CF): review of the literature. Rhinology 2011 Aug; 49(3): 347-355. 26. Koitschev A, Wolff A, Koitschev C et al.: Routine otorhinolaryngological examination in patients with cystic fibrosis. HNO 2006 May; 54(5): 361-364, 366, 368. 27. Eladl HM, Elmorsy SM: Endoscopic surgery in pediatric recurrent antrochoanal polyp, rule of wide ostium. Int J Pediatr Otorhinolaryngol 2011 Nov; 75(11): 1372-1375. 28. Maldonado M, Martines A, Alobid I, Mullol J: The antrochoanal polyp. Rhinology 2004; 43: 178-182. 29. Lee TJ, Huang SF: Endoscopic sinus surgery for antrochoanal polyps in child Otolaryngol Head Neck Surg 2006 Nov; 135(5): 688-692. 30. Rosbe KW, Jones DT, Rahbar R et al.: Endoscopic sinus surgery in cystic fibrosis: do patients benefit from surgery? Int J Pediatr Otorhinolaryngol 2001 Nov 1; 61(2): 113-119. 31. Yung MW, Gould J, Upton GJ: Nasal polyposis in children with cystic fibrosis: a long-term follow-up study. Ann Otol Rhinol Laryngol 2002 Dec; 111(12 Pt 1): 1081-1086.

