Karolina Raczkowska-Łabuda, Monika Jabłońska-Jesionowska, Jolanta Jadczyszyn, Magdalena Frąckiewicz, Maciej Pilch, *Lidia Zawadzka-Głos
Self-induced pneumoparotitis – a rare case report
Pneumoparotitis wywołany autogennie ? opis przypadku rzadkiego schorzenia
Department of Paediatric Otolaryngology, Medical University of Warsaw
Head of Department: Associate Professor Lidia Zawadzka-Głos, MD, PhD
Streszczenie
Pneumoparotitis (pneumoparotiditis, pneumosialoadenitis) to schorzenie rzadkie i często błędnie diagnozowane. Cechy odmy podskórnej w rzucie przyusznicy, szyi czy śródpiersia należą do niepokojących objawów, które bezzwłocznie należy wyjaśnić. Badanie TK stanowiące złoty standard diagnostyczny można w populacji dziecięcej z powodzeniem zastąpić badaniem USG. Sialografia stanowi dopełnienie obrazowania gruczołu i daje szansę na irygację przewodów ślinianki z zalegających złogów ? w prewencji nawracających stanów zapalnych. W artykule przedstawiono przypadek 12-latka, który świadomie, przez około pół roku, wypełniał obie przyusznice powietrzem, wytwarzając pneumocele prawego gruczołu o średnicy ok. 25 mm. Diagnostykę obrazową rozszerzono o badania z grupy chorób autoimmunologicznych. Przeprowadzono szczegółowy wywiad rodzinny. Zastosowano leczenie zachowawcze, w tym irygację przewodu Stenona deksametazonem, uzyskując pełne wycofanie objawów. W 3-miesięcznej obserwacji nie stwierdzono powiększenia pneumocele ani epizodu parotiditis.
Summary
Pneumoparotitis (pneumoparotiditis, pneumosialoadenitis) is a rare and frequently misdiagnosed condition. Signs of subcutaneous emphysema in parotid gland, the neck or the mediastinum are alarming symptoms that should be promptly addressed. Computed tomography, which is the gold diagnostic standard, may be successfully replaced with ultrasonography in the paediatric population. Sialography has a complementary role in the imaging of the gland, and allows for parotid duct irrigation to remove deposits and prevent recurrent inflammation. The paper presents a case of a 12-year-old boy who deliberately inflated his both parotid glands for about 6 months, and thus developed parotid pneumatocele with a diameter of about 25 mm. Diagnostic imaging was extended to include autoimmune diseases. Detailed family history was obtained. Conservative treatment, including dexamethasone irrigation of the Stensen’s duct, was used and the symptoms fully resolved. A 3-month follow-up showed no increase in the size of pneumatocele or episodes of parotiditis.
Introduction
The parotid glands should be impalpable in children. Noticeable facial asymmetry and unilateral or bilateral oedema are usually associated with parotid pathology. The most common causes include infection, autoimmune or lymphoproliferative diseases, duct obstruction, as well as benign or malignant tumours. Rapidly growing oedema is a typical outcome of viral infection or chronic, recurrent parotitis.
The term “pneumoparotid” refers to the presence of air in either the parotid gland or the Stensen’s duct (1, 2). Pneumoparotiditis is a parotitis secondary to a retrograde pathogen invasion through the duct orifice, which is dilated as a result of barotrauma (3). The presence of pneumoparotid in the absence of pneumoparotiditis is unlikely due to the diversity of oral flora. The co-existence of secondary inflammatory oedema often leads to a misdiagnosis of simple parotitis.
Patients at risk of pneumoparotitis include wind instrument players and adults with mental disorders (4, 5). Most patients are aware of their ability to inflate the gland. Patients with unintentional parotid gland inflation pose a greater diagnostic challenge.
Case report
A 12.5-year-old boy was admitted in an emergency to the Department of Paediatric Otolaryngology, Medical University of Warsaw, due to a swelling in the right cheek. About 1.5 days earlier, he completed a 7-day course of oral clindamycin for parotitis. The diagnosis and the treatment were suggested in the Department of Otolaryngology of another centre, where the patient had stayed for 5 days prior to outpatient treatment. At that time, salivary gland ultrasound showed inflammation of the gland and a cyst in the mandibular angle. Head CT was performed (fig. 1, 2).
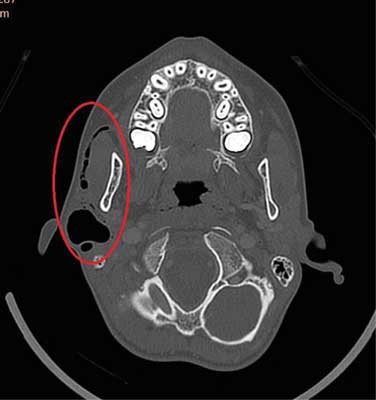
Fig. 1. CT in a patient with pneumoparotid. A dilated Stensen’s duct and a pneumatocele in its distal part may be seen on the right
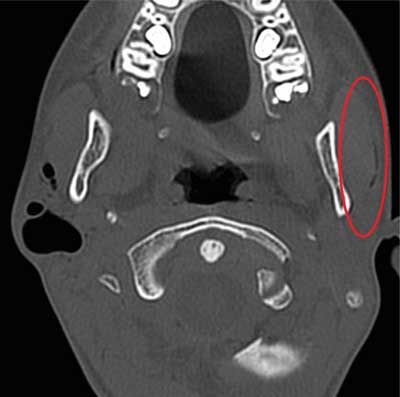
Fig. 2. CT in a patient with pneumoparotid. A dilated Stensen’s duct on the left
The findings were as follows: “A 23 mm space filled with air in the right parotid region, below the external auditory canal, in the projection of the salivary gland; air is also present in the salivary ducts of the right parotid gland and the parotid duct. On the left, air is present in the parotid duct and salivary ducts within the gland. Cervical lymphadenopathy up to 21 mm. Positive intra-oral pressure causes dilation of both the salivary ducts and the right-sided gas-filled space. The CT image most likely corresponds to a cyst drained through a fistula to salivary ducts, with a leaking orifice and air reflux into the salivary glands”. Buccal oedema located directly under the right ear lobe again increased rapidly, causing moderate pain in the child. Inflammatory markers on admission were normal: CRP < 0.5, WBC 8.59 thous., LYMF 34.3% (N: 24.7-56.0%), NEUT 56.3% (N: 43-65%), MONO 6.6% (N: 4.4-12.3), EOSINO 2.4% (N: 0.0-4.4%). Body temperature was 36.6°C. Otolaryngological examination showed no evacuation of pathological content from the dilated orifice of the gland, but only normal secretion containing air bubbles. Emergency ultrasound findings were as follows: “Both parotid glands are not enlarged, but show inhomogeneous parenchymal echostructure, without any focal lesions. A slight amount of air in the left parotid duct; a large amount of air and a small amount of fluid (about 6-5 cm) in the right parotid gland. Slightly increased vascularity in the right parotid gland. Mild reactive lymphadenopathy up to approx. 24 x 7 mm at both submandibular areas. No oedema of the surrounding tissue”. Based on the clinical picture and medical history, IV Amoxiclav was included by the doctor on duty. The boy admitted that during the past months he “played” by inflating both parotid glands. Initially, the dilation of the distal end of the duct was unnoticeable, and the resistance during gland insufflation was high. With time, however, the boy was able to develop a 27-mm-long pneumatocele. He also reported that he derived much joy from the “bubbling sensation” during air evacuation.
According to the patient’s mother, the boy had a history of 2 episodes of right-sided parotitis. Purulent discharge from the Stensen’s duct was observed each time. Both infections were developed in the last 6 months.
At the time of admission, the patient was under outpatient immunological, gastroenterological and cardiological care. He complained of a few-minute episodes of intra-abdominal pain unrelated to food ingestion for several years. His mother confirmed the lack of appetite, extended chewing time and difficulties swallowing solid foods. Percentile charts were difficult to develop due to the lack of measurements. At examination, the boy’s body weight of 31 kg corresponded to the third percentile (25th percentile at 5 years old), and his height of 149 cm corresponded to the 25th percentile (27th at 5 years old). The mother reported recurrent URTIs, allergy (no allergen test or treatment), gastroesophageal reflux disease (no medical records or treatment) and excluded celiac disease (again no records). Family history additionally revealed that the boy’s father was diagnosed with and treated for Barrett’s oesophagus.
Laboratory tests were run at the Department to evaluate total IgE, serum and urine amylase, anti-TPO, TSH, ANA, ANCA, Ig A, Ig M, Ig G, EBV, CMV, RF, CRP, and complete blood cell count. Abnormal findings were reported for total IgE ? 163 kU/L (N: 0-100). The above mentioned ultrasound was performed.
The patient was qualified for bilateral sialography. The procedure was performed under local anaesthesia: “Contrast agent (Imeron) was administered through a catheter inserted into the right parotid duct ? the dilated parotid duct was filled; several smaller spaces in the distal part of the duct and one significantly larger space were also filled. Contrast agent evacuation from these spaces was spontaneous (fig. 3). This was followed by contrast agent (Imeron) administration through a catheter inserted into the left parotid duct ? the parotid duct, which gave off branches in its distal dilated part, was filled with contrast agent (fig. 4)”.
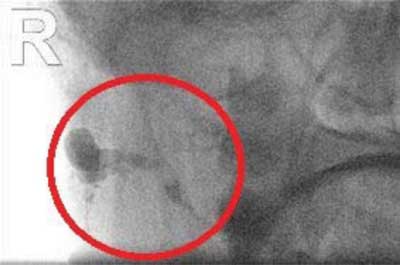
Fig. 3. Right parotid fistulography. Dilated duct and a contrast-enhanced cistern at its end are seen

Fig. 4. Left parotid fistulography. A dilated duct, mainly in its distal portion, is seen
A decision was made during the examination to qualify the patient for intraductal administration of dexamethasone. Bilateral irrigations were performed every day until the child declared distension-related pain. Treatment outcomes were monitored based on salivary gland ultrasonography and the decreasing volumes of drug that could be introduced without tissue resistance. Initially, 5 and 3 mL of solution were introduced into the right and the left duct, respectively. The treatment was completed after 6 days, when the radiologist reported a non-dilated right parotid duct (1.5 mL for the right and 0.5 mL for the left side) (fig. 5).
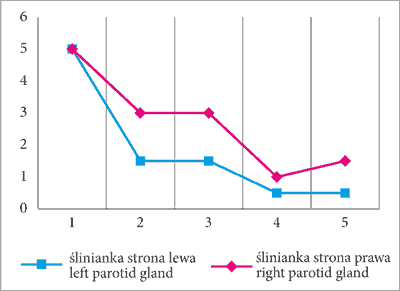
Fig. 5. Dexamethasone volumes administered in both parotid ducts
Ultrasound findings were as follows: “Both parotid glands of normal size, but showing inhomogeneous, abnormal parenchymal echostructure (more pronounced in the right gland), with no focal lesions. A lesion filled with air, 5 mm in diameter, and a duct (about 9 mm in length) filled with air, running from the ear lobe to the upper pole of the parotid gland, are present subcutaneously, near the upper pole of the right parotid gland. The parotid duct is not dilated. No parotid gland inflammation was detected. Minor reactive lymphadenopathy is present at both submandibular areas, more pronounced on the right side, up to about 21 mm, compared to the left side (14 mm). Periparotid tissues unremarkable. Submandibular salivary glands and the thyroid are normal”.
In cooperation with the doctors in the Department of Gastroenterology, the patient was qualified for gastroscopy with oesophageal biopsy to exclude eosinophilic esophagitis (EoE). Normal macroscopic and histopathological findings were obtained.
The 12-year-old boy was discharged the following day, instructed to follow intensive oral hygiene routine and incorporate acidic products in the diet. The boy was instructed to avoid intentional parotid insufflation, chewing gums, whistling and playing wind instruments. A 3-month follow-up showed no increase in the size of pneumatocele or episodes of parotitis.
Discussion
The following terms are used in literature to refer to the presence of air in the parotid gland: pneumoparotitis (6), pneumoparotiditis (7, 8), pneumosialoadenitis (9), surgical mumps or anaesthesia mumps (10), and pneumatocele glandulae parotis (11-13). Currently, the term pneumoparotitis is increasingly used, which does not indicate inflammation of the gland as opposed to pneumoparotiditis.
Pneumoparotid may be occupational or self-induced. Regardless of the aetiology, the condition is a consequence of increased intra-oral pressure and retrograde air flow into the Stensen’s duct and gland lobules. It may be either unilateral (11, 14, 15) or bilateral (5, 6, 16) ? often asymmetric, which was the case of our patient.
Self-induced cases of the disease among both children and adults are most common in literature (5, 6, 11, 12, 14, 15, 17-19). The problem usually occurs in the context of psychological and psychiatric disorders (4, 5, 20), traumatic events (22), nervous tics (14, 21), and a way to avoid school (5, 14) or even imprisonment (2). Cases of pneumoparotitis due to aggressive nose blowing (23), following spirometry (24), and sneezing with a blocked nose due to allergic rhinitis (25) have been described.
Under physiological conditions, the parotid gland is protected against retrograde invasion of pathogens, saliva or air by a system of mucosal folds covering the orifice, a slit-like shape of the orifice itself, the diameter of which is smaller than that of the Stensen’s duct, and a mechanism responsible for closing the orifice of the duct in response to increasing intra-oral pressure. The mechanism involves increasing duct angulation by compressing the anterior edge of the masseter muscle with the cheek stretched and a simultaneous closure of the orifice by the buccinator (5, 6, 14, 20). Therefore, an increase in the intra-oral pressure above typical values, i.e. 2-3 mmHg (up to 150 mmHg in trumpet players (26)), buccinator hypotonia, masseter hypertrophy, deposits in the Stensen’s duct impairing salivary flow, and dilated orifice (6, 14) promote pneumoparotid and pneumoparotiditis.
Pneumoparotitis most often manifests as salivary gland oedema with crepitation on palpation (3, 4). Foamy or purulent discharge from one or both parotid duct orifices may be observed (3, 4). Air bubbles coming from the duct are often observed during buccal palpation, which was also the case of our patient (3, 11, 12, 14). The skin over the gland may be warm and flushed. The tissue surrounding the gland does not have to be painful on palpation (27). Subcutaneous emphysema of the face and neck (3, 11, 21) and even pneumothorax (3, 5, 11, 28, 29) have been described in extreme cases. Sudden onset and short duration are typical of the disease (3, 18, 29). Additional history of blowing balloons, playing wind instruments or nervous ticks helps make the correct diagnosis. Computed tomography, which clearly differentiates between air and deposits/calculus in the Stensen’s duct, is the gold diagnostic standard (3, 4, 16). Recent research indicates that the imaging should be performed in a patient with inflated cheeks to increase sensitivity (3, 16). Ultrasonography may be performed as a preliminary study, especially in paediatric patients (3, 16, 29). Sialography usually shows a dilated Stensen’s duct (29, 30); if chronic, recurrent pneumoparotitis is present, ductal dilatation and characteristic “sausaging” may be observed (29, 30).
The treatment involves both conservative and surgical methods. Anti-inflammatory treatment and antibiotic therapy to prevent bacterial superinfection are sufficient for mild lesions. Psychologist or psychiatrist support may be needed to change unhealthy habits (5, 6, 14). Cheek compressors may be used in patients at an occupational risk of increased intra-oral pressure (3, 11, 19, 29).
Due to the short interval between recurrences in our patient, a decision was made to perform dexamethasone irrigation of the Stensen’s duct immediately after sialography and during the several days of in-patient follow-up. The aim of such management is to remove deposits, suppress inflammation and shrink the parotid duct (31-36). Ligation of the Stensen’s duct is the first-line surgical procedure. Parotidectomy is reserved for patients with high recurrence rates (2, 3, 4, 19).
Conclusions
Although pneumoparotitis is a rare entity, it should be included in the differential diagnosis of recurrent parotitis. Parotid ultrasonography is a commonly used diagnostic method in the paediatric population due to the typical clinical picture facilitating the diagnosis, wide availability and relatively low costs. Sialography irrigation of the duct with steroid solution may be considered in the prevention of recurrent inflammation in the pneumatocele. Patient’s cooperation is needed to eliminate the habit of self-insufflation. It should be noted that most patients require conservative treatment, and surgery should be used in the case of previous treatment failure.
Piśmiennictwo
1. Zuchi DF, Silveira PC, Cardoso C de O et al.: Pneumoparotitis. Braz J Otorhinolaryngol 2011; 77(6): 806.
2. House LK, Lewis AF: Pneumoparotitis. Clin Exp Emerg Med 2018; 5(4): 282-285.
3. McGreevy AE, O’Kane AM, McCaul D, Basha SI: Pneumoparotitis: a case report. Head Neck 2013; 35(2): E55-59.
4. McCormick ME, Bawa G, Shah RK: Idiopathic recurrent pneumoparotitis. Am J Otolaryngol 2013; 34(2): 180-182.
5. Balasubramanian S, Srinivas S, Aparna KR: Pneumoparotitis with subcutaneous emphysema. Indian Pediatr 2008; 45: 58-60.
6. Markowitz-Spence L, Brodsky L, Seidell G: Self-induced pneumoparotitis in an adolescent. Int J Pediatr Otorhinolaryngol 1987; 14: 113.
7. Hemphill RA: Wind parotitis. N Engl J Med 1973; 289: 1094.
8. Calcaterra TC, Lowe J: Pneumoparotitis: an unusual case of parotid gland swelling. Arch Otolaryngol 1973; 97: 468.
9. Brodie HA, Chole RA: Recurrent pneumosialadenitis: a case presentation and new surgical intervention. Otolaryngol Head Neck Surg 1988; 98: 350-353.
10. Reilly DJ: Benign transient swelling of the parotid glands following general anesthesia: “anesthesia mumps”. Anest Analg 1979; 49: 560-563.
11. Luaces R, Ferreras J, Patiño B et al.: Pneumoparotid: a case report and review of the literature. J Oral Maxillofac Surg 2008; 66: 362-365.
12. Martin-Granizo R, Herrera M, Garcia-Gonzalez D, Mas A: Pneumoparotid in childhood: report of two cases. J Oral Maxillofac Surg 1999; 57: 1468-1471.
13. Greisen O: Pneumatocele glandulae parotis. J Laryngol Otol 1968; 82: 447-480.
14. Gouguen LA, April MD, Karmody CS, Carter BL: Self-induced pneumoparotitis. Arch Otolaryngol Head Neck Surg 1995; 121: 1426-1429.
15. Faure F, Poulin Gaudon I, Tavernier L et al.: A rare presentation of recurrent parotid swelling: Self-induced parotitis. Int J Pediatr Otolaryngol 2009; 4: 29-31.
16. Lasboo AA, Nemeth AJ, Russell EJ et al.: The use of the “puffed cheek” computed tomography technique to confirm the diagnosis of pneumoparotitis. Laryngoscope 2010; 120: 967-969.
17. Meerleer KD, Hermans R: Images in clinical radiology: pneumoparotitis. JBR-BTR 2005; 88: 248.
18. Grainger J, Saravanappa N, Courtney-Harris RG: Bilateral pneumoparotid. Otolaryngol Head Neck Surg 2006; 134: 531-532.
19. Hann S, Isaacson G: Recurrent pneumoparotid: cause and treatment. Otolaryngol Head Neck Surg 2004; 131: 758-761.
20. Watt J: Benign swelling of parotid gland: a review. Proc Royal Soc Med 1970; 70: 483.
21. Gudlaugsson O, Geirsson AJ, Benediktsdottir K: Pneumoparotitis: a new diagnostic technique and a case report. Ann Otol Rhinol Laryngol 1998; 107: 356-358.
22. Prabhu SP, Tran B: Pneumoparotitis. Pediatr Radiol 2005; 38: 1144.
23. Birzgalis AR, Curley WA, Camphor CL: Pneumoparotitis, subcutaneous emphysema and pleomorphic adenoma. J Laryngol Otol 1993; 107: 377-379.
24. Kirsch DDM, Shinn J, Porzio R et al.: Pneumoparotid due to spirometry. Chest 1999; 116: 1475-1478.
25. Garber MW: Pneumoparotitis: an unusual manifestation of hayfever. Am J Emerg Med 1987; 5: 40-41.
26. Banks P: Nonneoplastic parotid swelling: a review. Oral Surg Oral Med Oral Pathol 1968; 25: 732-745.
27. Ghanem M, Brown J, McGurk M: Pneumoparotitis: a diagnostic challenge. Int J Oral Maxillofac Surg 2012; 41(6): 774-776.
28. Palau JR, Gonalez-Lagunas J, Linares JG: Self-induced parotid and parapharyngeal emphysema. Int J Oral Maxillofac Surg 2009; 38: 574.
29. Lagunas JG, Fuertes AF: Self-induced parapharyngeal and parotid emphysema: A case of pneumoparotitis. Oral and Maxillofacial Surgery Cases 2017; 3(4): 81-85.
30. Mandel L, Kaynar A, Wazen J: Pneumoparotid: a case report. Oral Surg Oral Med Oral Pathol 1991; 72: 22-24.
31. Premnath KPB, Thomas J, Ray B, Jayakrishnan V: Multimodality diagnostic features and treatment by sialography of juvenile recurrent parotitis: a case report. Int J Scientific Study 2016; 8(4): 176-178.
32. Zenk J, Schneider H, Koch M, Iro H: Current management of juvenile recurrent parotitis. Curr Otorhinol Rep 2014; 2: 64-69.
33. Narsimha Rao VV, Putta Buddi JS, Kurthukoti AJ: Juvenile recurrent parotitis in children: Diagnosis and treatment using sialography. J Indian Soc Pedod Prev Dent 2014; 32: 262-265.
34. Nahlieli O, Shacham R, Shlesinger M, Eliav E: Juvenile recurrent parotitis: A new method of diagnosis and treatment. Pediatrics 2004; 114: 9-12.
35. Gary C, Kluka EA, Schaitkin B, Walvekar RR: Interventional sialendoscopy for treatment of juvenile recurrent parotitis. J Indian Assoc Pediatr Surg 2011; 16: 132-136.
36. Ramakrishna J, Strychowsky J, Gupta M, Sommer DD: Sialendoscopy for the management of juvenile recurrent parotitis: A systematic review and meta-analysis. Laryngoscope 2015; 125: 1472-1479.




