© Borgis - New Medicine 4/2003, s. 112-116
K. Kujawski1, M. Koter1, P. Duchnowicz1, Kornelia Kedziora-Kornatowska2, Tomasz Kornatowski3, Leszek Szadujkis-Szadurski3, Jozef Kedziora4, Leszek Markuszewski1, R. Blaszczak1
The impact of Q10 on selected structural and functional parameters of red blood cells in old-age patients with primary hypertension
1 Department of Biophysics, University of Lodz, Poland
2 Department of Geriatrics, L.Rydygier Medical Academy of Bydgoszcz, Poland
Head: Kornelia Kedziora-Kornatowska, MD, PhD
3 Department of Pharmacology and Therapy, L.Rydygier Medical Academy of Bydgoszcz, Poland
Head: Leszek Szadujkis-Szadurski, MD, PhD
4 Department of Biochemistry, L. Rydygier Medical University of Bydgoszcz, Poland
Head: Jozef Kedziora, MD, PhD
Summary
The free radical theory of ageing assumes that in the older organism its oxidative balance gets shattered. Both arterial hypertension and oxidative stress cause structural and functional changes in the erythrocytes. Q10 is a compound which plays important roles in the cell and in its antioxidative properties, and may to a certain extent prevent unfavourable changes in the erythrocyte. Its amount in the human organism gradually decreases with age. The aim of this research was to evaluate membrane lipid peroxidation, inner microviscosity, and membrane ATPase activity in old-age normotensive and primary hypertensive patients before and after applying Q10.
The study was performed on 42 patients divided into three groups. The first two composed old-age patients with and without an accompanying hypertension. The third, control group consisted of healthy patients aged 51-59. In all three groups are shown the original parameters of red blood cells. In the case of elderly people the parameters were additionally recorded after three and six weeks of the Q10 application.
Membrane lipid peroxidation was found to be strongest in the both groups of elderly people, and the weakest in the control group. The application of Q10 resulted in a decrease in this parameter in both groups. The activity of total and Na+K+ dependent ATPase appeared the greatest in the control group, and the lowest in the group with hypertension. Q10 supplementing in the two groups caused the intensification of total and Na+K+ dependent ATPase activity. The highest value of inner microviscosity was observed in people with hypertension, and the lowest in the control group. After the application of Q10 it declined in both groups.
The results may point to a very beneficial effect of supplementary and metabolic therapy on structural and functional parameters in elderly people, especially with an accompanying arterial hypertension.
INTRODUCTION
The main reasons for mortality in elderly people are cardiovascular diseases (1, 2, 3), where one of the major risk factors is arterial hypertension. According to epidemiological data, 60 to 80% of people over 65 suffer from hypertension (4).
The free radical theory of organism ageing is based on the phenomenon of oxidative stress, i.e. pro- and antioxidative homeostasis disturbances contributing to generation of an increased reactive oxygen form (ROF) (5). The impact of ROF on the human organism is widespread and complex. Among others, the red blood cell is affected by increased membrane lipid peroxidation, aggregation of membrane proteins, and an increase in permeability or decrease in membrane deformation (6), all of which lead to structural and functional changes, shortening of their life and, as a result, compensated or decompensated anaemia (7). Consequent on the ROF activity, a direct or indirect effect may appear on the rise in susceptibility of large arterial vessels, endothelium functional disorder, or enhanced atheromatous processes, which may indicate their taking part in the pathogenesis of primary hypertension (8, 9). The main mechanisms responsible for protection from ROF are low molecular antioxidative enzymes (8). One of the low molecular antioxidants is Q10 (10, 11).
Q10 (2,3-dimetoxi-5-methyl-6-poliizoprenyl-bensochinon, international name – ubidekarenon) also referred to as ubichinon (ubitarius – widespread, chinon – cyclic compound consisting of two ketone groups) is chinon derived, similar to vitamins A, E and K. Q10 is a crucial compound in the existence of every cell. It is generated in all tissues and cells, from tyrosine and fatty acids, is fat-soluble, and takes two forms: oxidized (ubichinon) and reduced (ubichinol). Q10 is an integral part of the respiratory chain, playing the role of a mobile electron carrier from reduced coenzymes to cytochromes. Its reduced form (ubichinol) is an antioxidant with two-layered activity: direct organism protection against the harmful activity of free oxygen radicals, and indirect activity by the reduction of vitamin E to an active antioxidative form (10, 11, 12, 13, and 14). Q10 also influences the biosynthesis of ATP, cAMP (15).
Many researchers confirm the beneficial activity of Q10, among others, in cardiomyopathy, heart failure, ischaemic heart disease, hypertension, and arrhythmia (10). Moreover, it has been noted that Q10 in the human organism decreases in old age, and hence its supplementation in patients after 65 seems to be well-founded (10).
The research aimed at assessing the degree of membrane lipid peroxidation, the inner viscosity and membrane ATPase activity of red blood cells in elderly primary hypertensive and normotensive people before and after Q10 application.
MATERIAL AND METHODS
The subjects of the research were 20 people over 65 (average lifespan 71 ± 2) (group I – normotensive) and 11 patients over 65 with (average lifespan 69 ± 3) (group II – with mild and moderate hypertension according to Rapport VI JNC). The control group embraced 20 healthy people age 50 to 59. It is to be noted that smokers, people abusing alcohol, patients suffering from other cardiovascular diseases, secondary hypertension, diabetes, hypercholesterolaemia, neoplastic disease, overweight and obesity, as well as people taking medicines with known antioxidative properties were excluded from the research. The qualification of research subjects took place on the basis of: history and physical examination, body mass index, routine lab tests (full blood count, serum biochemistry, lipids profile, urinalysis), which were meant to discover earlier accompanying diseases likely to influence the oxidation-reduction organism balance as well as secondary hypertension. People in old age were given Q10 (medicines named: Envit, Polfa Pabianice) in a daily dose of 60 mg. Patients with hypertension were subjected to monotherapy with thiazide or thiazide-derived diuretics. Selected structural and functional parameters of red cells were assessed before the Q10 application and after 3 and 6 weeks of its use.
The inner viscosity of erythrocytes was examined by means of spin markers with the help of electron paramagnetic resonance (16, 17). The peroxidation degree of membrane lipids was defined by Stocks and Dormandy´s method using the reaction of the final peroxidation product of unsaturated fatty acids – malonic dialdehyde with tiobarbituric acid (TBA). The results point to the share of all compounds reacting with TBA-TBARS (18) in the test. The activity of total ATPase and Na+K+ dependent ATPase was tested by a method based on the measurement of released orthophosphate level, according to Bartosz (19, 20).
Statistical calculations were made with the help of the „Statistica” program and F-Fisher and T-Student tests.
RESULTS
The inner viscosity of erythrocytes was the lowest in the control group, medium in the group I and considerably the highest (from the statistic point of view) in the group II. The applied Q10 supplement, for 3 and 6 week, decreased in viscosity in groups I and II with statistically important value (after 6 weeks of supplementation) in the group II (chart I, table 1).
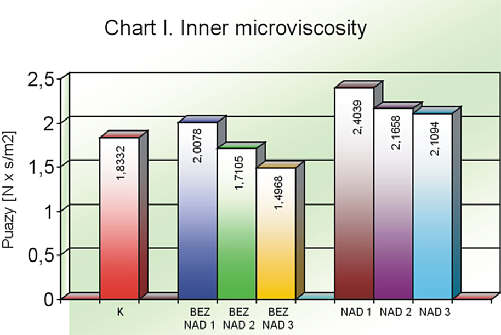
Chart I. Inner microviscosity
Explanations of charts I, II, III i IV:
Group names in use: K – control group; BEZNAD1 – group without hypertension before applying Q10; BEZNAD2 – group without hypertension after 3 weeks of Q10 application; BEZNAD3 – group without hypertension after 6 weeks of Q10 application; NAD1 – group with hypertension before applying Q10; NAD2 – group with hypertension after 3 weeks of Q10 application; NAD3 – group with hypertension after 6 weeks of Q10 application.
Table 1. Inner microviscosity of red blond cells in particular groups.
| | Mean value | Maximum value | Minimum value | Standard deviation |
| K | 1.8332 | 3.6310 | 1.2592 | 0.6008 |
| BEZNAD1 | 2.0048 | 3.9579 | 1.0457 | 0.7953 |
| BEZNAD2 | 1.7105 | 2.2993 | 1.2090 | 0.3321 |
| BEZNAD3 | 1.4968 | 2.19 | 1.2093 | 0.3134 |
| NAD1 | 2.4039* | 3.7997 | 1.878 | 0.5702 |
| NAD2 | 2.166 | 3.1997 | 1.6889 | 0.4200 |
| NAD3 | 1.9426** | 3.025 | 1.578 | 0.4483 |
* p <0.05 (NAD1 vs K), ** p <0.05 (NAD3 vs NAD1)
In comparison with the normotensive and control group, a statistically relevant TBARS concentration was observed in the people over 65 with primary hypertension. Q10 supplementation in aged patients with or without hypertension had a considerable influence on the lowering of TBARS concentration in both cases (chart II, table 2).
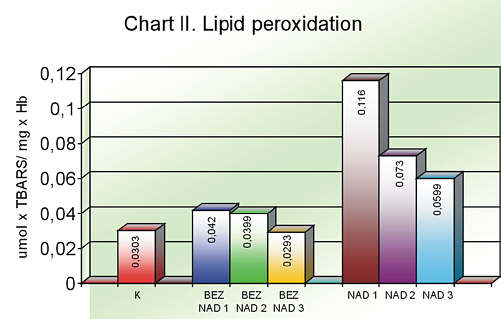
Chart II. Lipid peroxidation
Table 2. Lipid peroxidation (TBARS concentration) of red blood cells.
| | Mean value | Maximum value | Minimum value | Standard deviation |
| K | 0.0303 | 0.1192 | 0.007 | 0.0368 |
| BAZNAD1 | 0.0420 | 0.1698 | 0.0083 | 0.0498 |
| BEZNAD2 | 0.0339 | 0.0982 | 0.0081 | 0.0321 |
| BEZNAD3 | 0.0293 | 0.1002 | 0.0058 | 0.0301 |
| NAD1 | 0.1160* | 0.364 | 0.0159 | 0.1011 |
| NAD2 | 0.073 | 0.31 | 0.016 | 0.0862 |
| NAD3 | 0.0559** | 0.21 | 0.0185 | 0.0694 |
* p <0.05 (NAD1 vs K), ** p <0.05 (NAD3 vs NAD1)
The total ATPase activity was the greatest in the control group whereas the lowest was noted in the group of people over 65 with an accompanying hypertension, which constituted a statistically crucial difference. During the Q10 therapy the value of this parameter gradually increased. Taking into account the group with an accompanying hypertension, the activity growth was statistically marked (chart III, table 3).
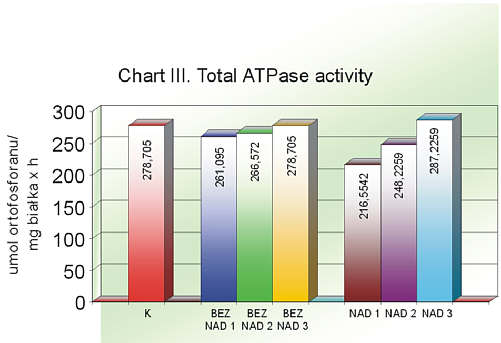
Chart III. Total ATPase activity
Table 3. Total ATPase activity in red blood cells.
| | Mean value | Maximum value | Minimum value | Standard deviation |
| K | 278.7050 | 447.49 | 125.73 | 77.0552 |
| BAZNAD1 | 261.0950 | 429.170 | 129.970 | 89.9174 |
| BEZNAD2 | 266.5720 | 488.09 | 134.89 | 101.5216 |
| BEZNAD3 | 284.5590 | 479.98 | 130.98 | 101.4386 |
| NAD1 | 216.5542* | 313.2631 | 156.1966 | 54.7187 |
| NAD2 | 248.250 | 389.9806 | 151.081 | 72.8777 |
| NAD3 | 287.2259** | 402.9143 | 191.1114 | 70.8337 |
* p <0.05 (NAD1 vs K), ** p <0.05 (NAD3 vs NAD1)
The Na+K+ dependent ATPase activity seemed similar to total ATPase activity with an exception to the original value which was slightly lower in the control group than in elderly people without an accompanying hypertension (chart IV, table 4).

Chart IV.Na+K+ dependent ATPase activity
Table 4. Na+K+ dependent ATPase activity in red blood cells.
| | Mean value | Maximum value | Minimum value | Standard deviation |
| K | 96.9510 | 180.41 | 24.05 | 45.9308 |
| BAZNAD1 | 101.4310 | 214.51 | 41.75 | 56.7866 |
| BEZNAD2 | 133.3820 | 212.34 | 59.92 | 63.6376 |
| BEZNAD3 | 157.2530 | 289.49 | 73.65 | 65.8211 |
| NAD1 | 64.0562* | 112.3804 | 22.3591 | 30.3347 |
| NAD2 | 85.1950 | 154.4527 | 22.3841 | 38.02687 |
| NAD3 | 127.9561** | 198.5913 | 84.5572 | 40.5713 |
* p <0.05 (NAD1 vs K), ** p <0.05 (NAD3 vs NAD1)
DISCUSSION
ROF can develop as a result of such external physical factors as ionized and ultraviolet radiation, ultrasound, or ROF photoreduction. Among other processes responsible for ROF emergence is oxidation of reduced forms of low molecular cell components in redox cycles and reactions of respiratory protein oxidation (21). They also come into being during certain non-enzymatic reactions, and, what is most important, in the process of changes taking place in the respiratory chain.
The growth in the speed of free radical appearance as well as the weakening of defence mechanisms lead to disorders in organism homeostasis, and an increase in the ROF impact on cells often produces irreversible effects.
The red blood cell is affected, among others, by enhancing the peroxidation of lipid membranes, membrane protein aggregation, and an increase in permeability or a decrease in membrane deformation (6). It leads to structural and functional changes of erythrocytes, shortening of their life, and subsequent to this, compensated or decompensated anaemia (7).
The consequence of ROF activity may be a direct or indirect influence on enhancing the susceptibility of big arterial vessels, impaired endothelium function and acceleration of atheromatous processes, which may indicate that they take part in pathogenesis of primary hypertension (8, 9).
One the ageing theories is also based on the phenomenon of oxidative stress, i.e. pro and antioxidative homeostasis disorder, leading to increased generation of oxygen forms (ROF) (5).
Internal research proved that peroxidation of lipid membranes gets stronger in old-age group. In addition to this, the ATPase activity becomes weaker and the inner microviscosity of red blood cell grows as compared to the control group. This may imply that oxidative stress increases with age.
In the available literature we find a double relation between oxidative stress and hypertension. On the one hand, oxidative stress is a relevant factor in the pathogenesis of hypertension (8, 9). However, on the other, it is reported that as a result of hypertension the human organism generates ROF more quickly.
The intensification of oxidative stress symptoms in patients suffering from hypertension was confirmed by internal research. An increase in lipid peroxidation, a decrease in ATPase activity and a rise in the inner microviscosity of red blood cells were recognised in people over 65 with primary hypertension, as compared with the control group and people over 65 without an accompanying hypertension.
Every living organism functions on variously-developed mechanisms which serve to protect against the harmful activity of free radicals in an enzymatic or a non-enzymatic way (25, 26, 27). Both a number of antioxidative enzymes, as well as low-molecular antioxidants, are ranked among them, e.g. carotene, vitamin A, vitamin C, vitamin E, glutathione, and Q10 (21, 25, 26).
Q10 in the human organism not only constitutes an integral part of the respiratory chain, in which it plays the role of a mobile electron carrier from reduced coenzymes to cytochromes. Its reduced form also possesses antioxidative properties. This is proved by a two-level activity: direct protection against the harmful activity of oxygen-free radicals and indirect influence through the reduction of vitamin E to an active antioxidative form. Q10 also exerts an impact on ATP and cAMP biosynthesis (15).
Our research shows that a supplement this compound improves structural and functional parameters of red blood cell in aged people, especially with an accompanying hypertension. It has been noted that membrane lipid peroxidation and inner microviscosity were reduced, and both total ATPase and Na+K+ dependent ATPase activity augmented. The results confirm the antioxidative properties of Q10. Other authors (12, 13, 14, 15) have also observed beneficial effects from a supplement of Q10 doses in cardiovascular diseases. However, we failed to find any research concerned with the effects of Q10 therapy in aged people and patients with an accompanying hypertension in the available literature.
CONCLUSIONS
The results show that oxidative stress becomes intensified with age. This is proved by an increase of membrane lipid peroxidation, a rise in inner microviscosity, and the curbed total ATPase and Na+K+ dependent AT-Pase activity of red blood cells. Similar results were collected in elderly patients with hypertension, as compared to the younger normotensive people. Q10 application in aged people had a beneficial influence on the tested structural and functional parameters of erythrocytes in the normotensive as well as in the hypertensive groups.
It has also been noted that the results may well point to the good impact of supplement-metabolic coenzyme Q10 therapy in old-age people, particularly where they have an accompanying hypertension.
Piśmiennictwo
1. Raszaja-Wanic B., Tykarski A.: Epidemiologia nadciśnienia tętniczego w wieku podeszłym. W: Tykarski A., red. Nadciśnienie tętnicze w wieku podeszłym. AM Poznań: Medica Press, 2000; 7-13. 2. Lewington S. et al.: Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903-1913. 3. Ogden L.G. et al.: Long-term absolute benefit of lowering blood pressure in hypertensive patients according to the JNC VI risk stratification. Hypertension 2000; 35:539-543. 4. Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure. (JNC-VI) Arch. Intern. Med. 1997; 157: 2413-2445. 5. Żakowska-Wachelko B.: Zarys medycyny geriatrycznej. Warszawa: Wyd. Lek. PZWL, 2000; 14-19. 6. Nohl H., Stolze K.: Formation of methemoglobin and free radicals in erythrocytes. Met. Ions Biol. Syst. 1999; 36:289-307. 7. Bartosz G., Erytrocyty W.: Dąbrowski Z., red. Fizjologia krwi. Warszawa: Wydawnictwo Naukowe PWN, 2000; 72-166. 8. Bartosz G.: Druga twarz tlenu. Warszawa: Wydawnictwo Naukowe PWN, 2003; 242-264. 9. Zalba G. et al.: Oxidative stress in arterial hypertension role of NAD(P)H oxidase. American Heart Association 2001; 1395-1399. 10. Drzewoski J.: Farmakologia kliniczna koenzymu Q10. Przegląd Lekarski. 1988; 45, 9:680-683. 11. Jones K. et al.: efficacy, safety, and use. Alternative therapies 2002; 8:42-56. 12. Thomas S.R. et al.: Dietary Cosupplementation with vitamin E and coenzyme Q10 inhibits atherosclerosis in apolipoprotein E gene Knockout Mice. Arterioscler Thromb. Vasc. Biol. 2001; 21:585-593. 13. Zhou M. et al.: Effects of coenzyme Q10 on myocardial protection during cardiac valve replacement and scavenging free radical activity in vitro. The Journal of cardiovascular surgery 1999; 40: 355-61. 14. Kukliński B. et al.: Coenzyme Q10 and antioxidants in acute myocardial infarction. Proceedings of the 8th International Symposium on the Biomedical and Clinical Aspects of Coenzyme Q10. Stockholm 1993. 15. Okamoto T. et al.: Biochemical role of coenzyme Q10 on cultured ventricular myocytes: effect of Q10 on mitochondrial ATP production. Proceedings of the 8th International Symposium on the Biomedical and Clinical Aspects of Coenzyme Q10. Stockholm, 1993. 16. Koter M.: Elektronowy rezonans paramagnetyczny. W: Jóźwiak Z., red. Materiały do ćwiczeń z biofizyki. Łódź: Wydawnictwo Uniwersytetu Łódzkiego 1993; p. 138-151. 17. Lowry O.H. et al.: Protein measurament with the Folin Phenol reagent. J. Biol. Chem. 1971; 20:265-275. 18. Stock J., Dormandy T.L.: The autooxidation of human red cell lipid-induced hydrogen peroxide. Brit. J. Haemat. 1971, 20:95-101. 19. Bartosz G. et al.: Stimulation of erythrocyte membrane Mg2+-ATPase by membrane disturbing agents. Biochem. Mol. Biol. Int. 1994; 34:521-529. 20. Veldhoven von P.P., Mannaerts G.P.: Inorganic and organic phosphate measurements in the nanomolar range. Anal. Bioch. 1987; 161:45-48. 21. Fu M.X. et al.: Role of oxygen in cross-linking and chemical modification or collagen by glucose. Diabetes 1992; 41:42-48. 22. Pawlak W. et al.: Effect of long-term bed rest in men on enzymatic antioxidative defence and lipid peroxidation in erythrocytes. Gravit. Physiol. 1998; 5:163-164. 23. Ceriello A. et al.: Vitamin E reduction of protein glycosylation in diabetes. New prospect for prevention of diabetic complications? Diabetes Care 1991; 14:68-72. 24. Halliwel B.: How characterize a biological antioxidant. Free Rad. Res. Comm. 1990; 9:1-32. 25. Halliwel B. et al.: The characterization of antioxidants. Food Chem. Toxicol. 1995; 33:601-617. 26. Fiohe L. et al.: Enzymatic defence systems against hydroperoxides and oxygen-centered radicals in mammals; glutathione peroxidases and superoxide dismutases, in the role of oxygen radicals in cardiovascular disease. Abbate A.L., Ursini F., Kulwer Acad. Publish. 1998; 231-243. 27. Meister A.: Glutathione metabolism and its selective modification. J. Biol. Chem. 1988; 263:17205-17208.



