© Borgis - New Medicine 4/2014, s. 118-121
Małgorzata Dębska, Elżbieta Niemczyk, *Lidia Zawadzka-Głos
Evaluation of drug-resistant Streptococcus pneumoniae in patients treated in Pediatric Hospital of Warsaw Medical University in 2012-2013
Department of Pediatric Otolaryngology, Medical University of Warsaw, Poland
Head of Department: Lidia Zawadzka-Głos, MD, PhD
Summary
Introduction. Streptococcus pneumoniae is the most common pathogen causing respiratory tract infections and invasive infections. In 2012 and 2013 we observed an increased incidence of drug-resistant SP in patients treated in Pediatric Hospital of Warsaw Medical University.
Aim. To evaluate the pneumococcal infections and the susceptibility to antibiotics. To examine the correlation between the incidence of pneumococcal infection and the use of pneumococcal vaccines.
Material and methods. The study included 38 children hospitalized due to pneumococcal infection in 2012 and 2013 in Pediatric Hospital of Warsaw Medical University. Basic information about microbiological examination, previous vaccine administration and hospitalization were gathered retrospectively and analyzed.
Results. Forty four specimens were obtained from 38 patients. Body culture sites were as follows: middle ear, nasal cavity sinuses, conjunctiva, pharynx. The drug-resistant strains were found in 12 cases. The patients suffered from meningitis, acute otitis media and rhinosinusitis. Isolates from 26 patients were described as susceptible. The infections developed as: acute otitis media, rhinosinusitis and pneumonia. Pneumococcal conjugate has been applied to 8 children before the hospitalization. Twenty two children had not been previously vaccinated. One out of 8 all vaccinated patients suffered from drug resistant pneumococcal infection. Children without active immunization developed drug resistant infection in 10 cases.
Conclusions. Pneumococcal infections occur less frequently in children with previous vaccine administration. Drug resistant isolates are more frequently responsible for invasive pneumococcal disease. Pediatric otolaryngologists recommend active immunization against Streptococcus pneumoniae.
INTRODUCTION
Streptococcus pneumoniae (SP) is a leading cause of lower and upper respiratory tract infections. (1, 2). Invasive pneumococcal disease (IPD) is defined as an infection in normally sterile body sites such as blood and cerebrospinal fluid (e.g. bacteremic pneumonia, bacteremia, meningitis) (3, 4). Individuals aged < 2 and > 65 years old are at particularly high risk for IPD (1). A heptavalent pneumococcal conjugate vaccine (PCV7) was licensed in the United States in 2000 and recommended for all children aged 2 to 23 months (3). It covered pneumococcal serotypes responsible for most antibiotic–resistant infections. In 2006 Kyawet al. (5) proved that introducing PCV7 into the routine childhood immunization program decreased the incidence of antibiotic-resistant IPD. An increasing in infections caused by serotypes not included in the vaccine was also observed. In 2009 higher-valent pneumococcal conjugate vaccines became available (6). They comprise additional 3 (PCV10) and 6 (PCV13) serotypes and are available in Poland since April 2009 and January 2010 (7, 8). In 2012 and 2013 we observed an increased incidence in drug-resistant SP in patients treated in Pediatric Hospital of Warsaw Medical University.
MATERIAL AND METHODS
A retrospective study was conducted with patients treated in Pediatric Hospital of Warsaw Medical University in 2012 and 2013. Several patients were selected based on SP isolates found in microbiological examination. Informations about the pneumococcal susceptibility to different classes of antibiotics were analyzed. Information about body culture site, department of hospitalization, gender, age of the child at the time of hospitalization, prior administration and type of pneumococcal vaccine, primary disease and comorbidities, concomitant infections, antimicrobial therapy were gathered and analyzed.
RESULTS
SP accounted for 1.21% (45/3723) of all isolates. These were obtained from 38 patients. One patient was hospitalized twice within 1 month due to pneumococcal infection. Both results were analyzed. In 4 cases samples were taken from two different body sites during one hospitalization. A body culture site less correlated to primary disease was excluded from analysis. Three patients were excluded due to incomplete data.
SP was found in 18 girls (47.37%) and 20 males (52.63%). These were children aged 3 weeks to 15 years with an average age of 44.1 months. Children younger than 2 years old constituted 44,74% of all hospitalized patients.
The samples were collected mainly from pediatric otolaryngology department. The group constituted 52,63% of hospitalized children due to pneumococcal infection (fig. 1). The most commonbody culture sites were as follows: ears (n = 16), and nose withparanasal sinuses (n = 11). They accounted for 38.1% and 26.19% of all analyzed samples (n = 42) (fig. 1).
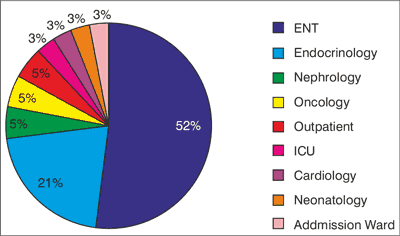
Fig. 1. Department of hospitalization of patients suffering from pneumococcal infections.
Susceptible SP were identified in 26 patients (68.42%). They caused mostly rhinosinusitis (RS) (n = 8) and acute otitis media (AOM) (n = 8). Two cases of AOM were complicated by acute mastoiditis (fig. 2).
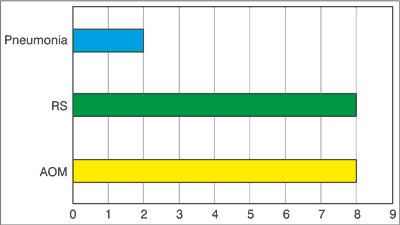
Fig. 2. The infections caused by susceptible strains.
RS – rhinosinusitis, AOM – acute otitis media
Antibiotic-resistant SP were found in 12 patients (31.58%) and caused mainly AOM (n = 9), including one case complicated by acute mastoiditis and meningitis (fig. 3).
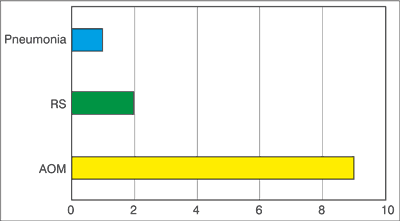
Fig. 3. The infections caused by resistant strains.
RS – rhinosinusitis, AOM – acute otitis media
Seven children suffered from systemic noninfectious diseases. These were: CHARGE syndrome, prune belly syndrome, immunodeficiency, pre-B cellacute lymphoblastic leukemia (ALL) and T cell ALL, hydronephrosis, aortic and mitral valve stenosis. One child died during hospitalization due to Escherichia coli septicemia.
Eight (21.05%) children received at least one dose of pneumococcal vaccine before the hospitalization. The PCV13 was administered to all of them. 22 (57.1%) children have not 0been previously vaccinated. In 8 cases (21.05%) the data about vaccination remained unknown. Microbiological examination found only one (12,5%) drug-resistant SP among children with previous PCV13 administration. 7 other cases (87.5%) revealed susceptible isolates. Drug-resistant SP were present in 12 (54.55%) children with no previous pneumoccocal vaccine administration. Other 10 unvaccinated patients (45.45%) had susceptible isolates in microbiological examination.
In our material SP isolates were resistant mainly to clindamycin: 11 resistant (R) cases and 1 intermediate resistant (I) case. Drug resistance was found also in following antibiotics: erythromycin (11R, 0I), trimethoprim-sulfamethoxazole (9R, 0I), penicillin (4R, 3I), ceftriaxone (1R, 5I); cefotaxime (1 R, 1I). If a SP isolate was resistant to penicillin, so it was to clindamycin, erythromycin, and trimethoprim-sulfamethoxazole. One isolate was susceptible to penicillin, but it was resistant to clindamycin, erythromycin and intermediate resistant to cefotaxime. Penicillin-resistant strains were microbiologically examined and revealed susceptibility (S) to:meropenem (3S), vancomycin (3S), levofloxacin (7S), chloramphenicol (5S).
The most common antimicrobial agent used during hospitalization were: cefuroxime (n = 8), amoxicillin with clavulanate (n = 8) and clindamycin (n = 8). Ceftriaxone (n = 4) and cefotaxime (n = 4) were used rarely. Clindamycin was administered always as a multidrug therapy with 2nd or 3rd generation of cephalosporin. A multidrug therapy was applied to treat severe infections in the course of leukemia. It consisted of: tazobactam and amikacin orimipenemcilastatin and amikacin. One case of meningititis was treated withmeronem and vancomycin.
DISCUSSION
The overall incidence of IPD in Europe in 2010 was 5.2 cases per 100 000 population (6). According to World Health Organization the incidence of IPD in Poland in the individuals aged 0-2 years is 19 cases per 100 000 population (9). Individuals < 2 and > 65 years of age are especially vulnerable to IPD and have the highest incidence and case fatality rate (6). So are patients with other risk factors such as:asplenia or splenic dysfunction, chronic respiratory disease, chronic heart disease, chronic kidney disease, chronic liver disease, diabetes, immunosuppression, cochlear implantation, cerebrospinal fluids leak (1). The mortality is still high and has not changed significantly in the past decades. The IPD-induced healthcare costs are vast (11). Clinical manifestations of IPD in neonates and young infants are serious and can include death (3). Our study shows no mortality due to pneumococcal infection. 44.74% of all hospitalized children were younger than 2 years old. 7 children presented comorbidities such as: cardiovascular, hematological and nephrological disorders. PCV7 was introduced in 2000 in the United States and since that time the burden of pneumococcal diseases in children has significantly decreased. So were an antibiotic resistance in vaccines serotypes and nasopharyngeal carriage of SP (12). A herd effect (indirect effect in unvaccinated populations) was also proved (13). This exemplifies by declines in IPD cases within adults aged > 65 years and infants < 60 days of age. A rise in serotype not included into PCV7 (serotype 19A) has been observed globally. This situation led to the development of higher-valent conjugate vaccines (3, 6).
Two kinds of pneumococcal vaccines are currently available in Poland: polysaccharide vaccines which comprise 23 serotypes (available for more than 20 years) and conjugate vaccines comprising 7, 10 or 13 serotypes (PCV7, PCV10 and PCV13) (9). The latter have more immunogenic power than the polysaccharide vaccines (11). PCV13 has been administered to 8 vaccinated patients before an onset of infection. Among them only one patient was infected by drug resistant SP. This is concomitant to other studies and confirms that drug resistant SP occur more frequently in unvaccinated individuals.
PCV10 is applied for children from 6 weeks up to 5 years of age. PCV 13 is administered in individuals from 6 weeks up to 17 years of age. Both vaccines are indicated in Europe for an active immunization against: IPD, AOM and pneumonia. Additionally, PCV13 can be applied in people older than 18 years of age against IPD (6).
A local analysis of SP drug resistance plays an important role in making therapeutic decisions. An increasing prevalence of penicillin resistant (PRSP) isolates is concerning (14). Tang et al. (15) has shown in 2002 that 23% of PRSP isolates were resistant to at least three antimicrobial classes, and 5 % of isolates were resistant to at least five antimicrobial classes. This percentage has been confirmed in study. In 2008 in Poland 70.7 % of pneumococcal isolates were susceptible to penicillin. Susceptibility to clindamycin accounted for 75.6%, macrolides – 72%, trimethoprim-sulfamethoxazole – 55.2% (14) .
Infections caused by SP can still be treated using beta-lactams (first-line antibiotic). The dosage should be optimized to cover less susceptible strains (16). Children from our hospital were administered mainly amoxicillin with clavulanate or cefuroxime with good therapeutic effect.
CONCLUSION
Pneumococcal infections occur less frequently in children with previous vaccine administration. Drug resistant isolates are more frequently responsible for invasive pneumococcal disease. Pediatric otolaryngologists recommend active immunization against Streptococcus pneumoniae.
Piśmiennictwo
1. Rodenburg GD, de Greeff SC, Jansen AGCS et al.: Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 2010; 16: 816-823. 2. Che D, Zhou H, He J, Wu B: Modeling the impact of the 7-valent pneumococcal conjugate vaccine in Chinese infants: an economic analysis of compulsory vaccination. BMC Health Services Research 2014; 14: 56 http://www.biomedcentral.com/1472-6963/14/56. 3. Poehling KA, Talbot TR, Griffin MR et al.: Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA 2006; 295: 1668-1674. 4. Kellner JD, Church DL, MacDonald J et al.: Progress in the prevention of pneumococcal infection. CMAJ 2005; 173: 1149-1151. 5. Kyaw MH, Lynfield R, Schaffner W et al.: Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354: 1455-1463. 6. Torres A, Bonanni P, Hryniewicz W et al.: Pneumococcal vaccination: what have we learnt so far and what can we expect in the future? Eur J ClinMicrobiol Dis. [Epub ahead of print] Available online at: http://download.springer.com/static/pdf/285/art%253A10.1007%252Fs10096-014-2208-6.pdf?auth66=1411413266_f50497a2f1f440eea18d5f5780f36b34&ext=.pdf. Accessed 23 August 2014. 7. Filipek J: Nowe rejestracje i nowości na rynku. Nowości na rynku – kwiecień 2009. Aptekarz Polski 2009; 33: 60-61. 8. Filipek J: Nowe rejestracje i nowości na rynku. Nowości na rynku – styczeń 2010. Aptekarz Polski 2010; 42: 53-55. 9. Rekomendacja nr 53/2014 z dnia 24 lutego 2014 r. Prezesa Agencji Oceny Technologii Medycznych. Available online at: http://www.aotm.gov.pl/bip/assets/files/zlecenia_mz/2013/351/REK/RP_53_2014_Synflorix.pdf. 10. van Hoek AJ, Andrews N, Waight PA et al.: The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J infect 2012; 65: 17-24. 11. Welte T: How can we reduce mortality of invasive pneumococcal disease? Eur Respir Rev 2012; 21: 6-7. 12. O’Brien KL, Millar EV, Zell ER et al.: Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. JID 2007; 196: 1211-1220. 13. Davis SM, Deloria-Knoll M, Kassa HT, O’Brien KL: Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 2014; 32: 133-145. 14. Domagała M, Ciebiada M: Pozaszpitalne zapalenia płuc u osób w wieku podeszłym. Geriatria 2010; 4: 252-258. 15. Tang YW, Li H et al.: Rapidly increasing prevalence of penicillin-resistant Streptococcus pneumonia in Middle Tennessee: a 10-year clinical and molecular analysis. J Clin Microbiol 2002; 40: 395-399. 16. Hryniewicz W, Ozorowski T, Radzikowski A et al.: Rekomendacje postępowania w pozaszpitalnych zakażeniach układu oddechowego 2010.


